Cerebral visual impairment (CVI) is a decreased visual response due to a neurological deficit along the visual pathway or the visual center of the brain. Usually, a child with CVI has normal eye findings or an eye condition that cannot explain the degree of visual disturbances. The most common etiology for CVI is perinatal hypoxia, cerebral vascular accident, meningitis, encephalitis, acquired hypoxia, hydrocephalus, or prematurity. Other less common etiologies included intracranial cyst, head trauma, or brain tumors.
Specifically, cortical visual impairment (CVI) denotes vision loss from diminished signaling along the retro-geniculostriate visual pathway (marked E, D, and F) in the absence of other ocular pathology.
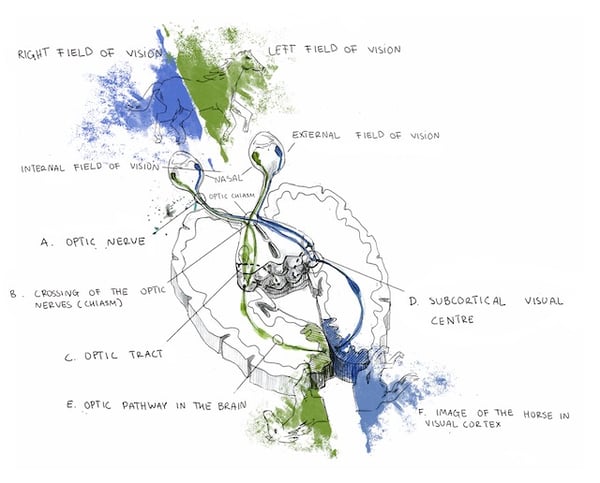 This condition is occasionally more broadly referred to as cerebral, rather than cortical in nature, as reduced vision may occur due to abnormalities in the subcortical optic radiations or occipital cortex. The incidence of CVI has been steadily rising over the past few decades and currently around 160 cases per 100,000 individuals. Worldwide, CVI is a major contributor to childhood vision loss. Visual function is diminished in CVI due to reduced or absent signaling from the eye to the brain. The degree of visual impairment ranges from mild to complete vision loss, called cortical blindness.
This condition is occasionally more broadly referred to as cerebral, rather than cortical in nature, as reduced vision may occur due to abnormalities in the subcortical optic radiations or occipital cortex. The incidence of CVI has been steadily rising over the past few decades and currently around 160 cases per 100,000 individuals. Worldwide, CVI is a major contributor to childhood vision loss. Visual function is diminished in CVI due to reduced or absent signaling from the eye to the brain. The degree of visual impairment ranges from mild to complete vision loss, called cortical blindness.
Children with CVI present with reduced vision, normal pupil responses, no nystagmus, and relatively normal ocular findings, although optic disc pallor may present later (quite rare). These children oftentimes have hyperopic (farsighted) refractive errors, strabismus (eye turns), and visual field defects (usually bilateral inferior defects or homonymous hemianopia). One unique feature of CVI sometimes observed is dyskinetic strabismus where an eye turn may switch between exotropia (outward) and esotropia (inward) sporadically. While nystagmus is absent in cases of cortical damage, a nystagmus may be present if the damage is subcortical at the level of the optic radiations.
Perinatal Hypoxia-Ischemia
CVI has many causes, but the most common in children is a perinatal hypoxic episode (asphyxia). Location of ischemic damage is dependent on infant age and term (premature vs full-term) due to the location of the cerebral watersheds at that developmental time. In preterm infants, infarctions occur in the periventricular deep white matter causing periventricular leukomalacia and affecting the optic radiations. Parasagittal infarctions are rare in preterm infants due to bridging of the meningeal anastomosis between the watershed zone and major cerebral arteries. In full-term infants, the watershed areas lie in the regions between the anterior and middle cerebral arteries as well as the medial and posterior cerebral arteries. At this stage of development, hypoxia-induced hypoperfusion to the watershed areas leads to infarction to the frontal, temporal, parietal, and occipital brain areas. Generally, insult to the optic radiations, rather than the cortical areas, has a worse prognosis. Ischemic brain damage may occur before or after birth, delaying dendrite formation and synaptogenesis, causing abnormal myelination of visual pathways, or leading to cell death of glial and neural cells due to impaired protein synthesis. Improved visual function in children with CVI may be due to rerouting of axons, reduced axon retraction, and reactive synaptogenesis.
Postnatal Hypoxia-Ischemia
Although most cases of CVI occur due to perinatal hypoxia, ischemic damage during infancy or later may also cause CVI. Postnatal hypoxia-ischemic brain injury along the visual pathway may occur due to cardiac arrest, respiratory distress, generalized hypotension, hypertensive crisis induced cerebral occlusion, transtentorial herniation compressing cerebral arteries, cardiac surgery, or air embolisms. Vascular malformations or central nervous system tumors may compress cranial vessels causing CVI. Congenital cyanotic heart disease may cause emboli formation and thrombotic disorders may arise from trauma, meningitis, polycythemia, neurofibromatosis, and sickle cell disease. Any number of conditions inducing cerebral ischemic injury may cause CVI during infancy.
Periventricular and Intraventricular Hemorrhages
CVI may occur from ischemic damage due to intracranial hemorrhages, which commonly occur in premature infants within the first few days of life. Such hemorrhages occur either spontaneously or from a hypoxic crisis in small vessels within the ventricular walls due to inadequate vascular support. Following an ischemic incident, reperfusion of these weak blood vessels may cause a hemorrhage. Hydrocephalus may ensue from these hemorrhages, dissecting the brain parenchyma and inflicting damage to the visual pathways. Also common in preterm infants are choroidal plexus hemorrhages which may cause subsequent periventricular leukomalacia and CVI.
Cerebral Malformations
CVI and congenital homonymous hemianopia may occur due to a multitude of cerebral malformations including: occipital encephaloceles, parietal encephaloceles, hydranencephaly, Chiari malformations, Dandy-Walker complex, porencephalic cysts, or neuronal migration abnormalities. Porencephalic cysts may occur from vascular compromise, infections, or hemorrhagic dissection while neuronal migration abnormalities may occur due to infections, ischemia, or metabolic disturbances. Migrational abnormalities result in normal neurons in abnormal locations. Clinical manifestations of these anomalies depend on the extent, timing, and location of migration which cause different categories of migrational abnormalities. The most severe migrational abnormality is lissencephaly which includes agyria and pachygyria. Other categories include neuronal heterotopias, unilateral megalencephaly, schizencephaly, porencephaly, and encephalomalacia.
Pediatric brain tumors and vision
 Solid brain tumors develop in approximately 30 out of 1,000,000 children and have a 10 year survival rate of just under 60%. Diagnosis is made through both clinical history and neuroradiological imaging and confirmed through histology. Despite the rare diagnosis and survival rate, brain tumors are the leading cause of cancer deaths in children. These tumors can cause significant visual impairments if the tumor is located in areas of the visual system or if the tumor leads to hydrocephalus (elevated intracranial pressure). Such visual impairments may negatively affect a childs everyday activities, development, education, and vocation if not treated effectively or in a timely manner.
Solid brain tumors develop in approximately 30 out of 1,000,000 children and have a 10 year survival rate of just under 60%. Diagnosis is made through both clinical history and neuroradiological imaging and confirmed through histology. Despite the rare diagnosis and survival rate, brain tumors are the leading cause of cancer deaths in children. These tumors can cause significant visual impairments if the tumor is located in areas of the visual system or if the tumor leads to hydrocephalus (elevated intracranial pressure). Such visual impairments may negatively affect a childs everyday activities, development, education, and vocation if not treated effectively or in a timely manner.
Symptoms of Brain Tumors in Children
Due to the brain tissue already fitting tightly inside the enclosed skull, tumor growth causes increased pressure within the skull, termed elevated intracranial pressure or hydrocephalus. Elevated intracranial pressure occurs mostly due to excess cerebrospinal fluid. This excess pressure exerted on the surrounding brain tissue, if severe, causes neurological dysfunction manifesting as disturbed vision, poor balance and coordination, impaired motor function, headaches, cognitive impairments, nausea and vomiting, irritability, drowsiness, or seizures. Symptoms are dependent on the location and size of the tumor as well as the degree of elevated intracranial pressure.
Brain Tumor Diagnosis in Children
Symptoms of brain tumors which locate close to visual pathways or visual centers often manifest initially as vision problems. For this reason, eye care professionals may be the first healthcare providers notified of tumor-related symptoms. Although not always, signs of brain tumor formation may be visible in the eye as optic nerve edema. Regardless of the presence of ocular signs, children experiencing brain tumor symptoms should be thoroughly and immediately evaluated by a pediatric neurologist, pediatrician, or emergency room physician.
 Neurological examination and neuroimaging are the first steps taken to confirm or deny the presence of an intracranial process as a tumor. Multiple scan types may be necessary to confirm the presence or absence of a tumor depending on the size, location, and type of tumor. Common neuroimaging methods include:
Neurological examination and neuroimaging are the first steps taken to confirm or deny the presence of an intracranial process as a tumor. Multiple scan types may be necessary to confirm the presence or absence of a tumor depending on the size, location, and type of tumor. Common neuroimaging methods include:
- Magnetic Resonance Imaging (MRI) ” creates detailed three-dimensional images of tissue by utilizing a strong magnetic field and the natural magnetic properties of human tissue.
- Magnetic Resonance Spectroscopy (MRI Spect or MRS) ” displays images similar to an MRI but also provides information on brain function and patterns of activity through the measurement of metabolites and cerebral blood flow. This is useful in assessing the aggressiveness of a tumor and its response to treatment.
- Perfusion MRI ” examines tissue blood flow and perfusion to assess the tumor grade and aggressiveness.
- Functional MRI ” visualizes oxygen use and blood flow to brain areas as the subject performs tasks. This is useful in identifying and evaluating areas of the brain responsible for visual, language, motor, and sensory functions which is helpful for surgery planning.
- Positron Emission Tomography (PET scan) ” utilizes small amounts of radioactive materials to visualize and map hypermetabolic activity indicative of abnormal tumor cells or scar tissue.
- Computed Axial Tomography (CAT or CT scan) ” displays a combination of bone, soft tissue, and blood vessels through a series of x-ray scans. This is typically the initial scan performed in emergent situations.
Brain tumors are classified by a standard created by the World Health Organization. This classification is characterized by the tumor cell type and location. Tumor types are differentiated by the cell type involved and the tumor grade represents the aggressiveness of the cells. Tumor grade ranges from 1-4 and is usually denoted in Roman numerals. The tumor grade is essential for treatment determination and prognosis predictions. High grade tumors (III and IV), grow quicker, inflict more damage, are more difficult to treat, and have worse survival rates than low grade tumors (I and II). High grade tumors are considered more malignant or cancerous. Conversely, low grade tumors progress slowly, inflict minimal damage, and have high long-term survival rates. In tumors, the most malignant cell present determines the grade for the tumor as a whole. Certain tumors may become malignant over time and change growth patterns.
Types of Brain Tumors in Children
- Primary: Initially forming in the brain
- Metastatic: Spread to the brain from another area of the body
- Benign: Non-cancerous and slow-growing tumor. Easier to treat than malignant, but still may be difficult depending on the location in the brain.
- Malignant: Cancerous and aggressive. Rapidly growing tumors which may spread to other areas of the brain or body.
Primary brain tumors (PBTs) may be subdivided into three groups: medulloblastomas, ependymomas, and astrocytomas. This classification is determined by the tissue origin, location, and histopathological characteristics. Medulloblastomas, accounting for approximately 20% of PBTs, are the most common malignant childhood brain tumors and are neuroectodermal tumors of the cerebellum. Ependymomas are the third most common PBTs of the CNS and oftentimes arise in the parenchyma of the cerebral hemispheres. Astrocytomas account for roughly 50% of all brain tumors in children and are the most common type of glioma. Four main types of astrocytomas exist in children:
- Pilocytic astrocytoma (grade 1): These are the most prevalent brain tumors in children and are slow-growing in nature and oftentimes fluid-filled (cystic). When developed in cerebellum they may be surgically removed.
- Diffuse astrocytoma (grade 2): This tumor may cause seizures is typically difficult to remove surgically due to infiltration of surrounding normal brain tissue.
- Anaplastic astrocytoma (grade 3): This is a malignant brain tumor with varying symptoms depending on the location. A combination of treatments is generally utilized.
- Glioblastoma multiforme (grade 4): This is a rapidly growing, highly malignant tumor requiring combination treatments. This tumor type may also cause increased intracranial pressure.
Other Pediatric Brain Tumors
Brain stem gliomas: The majority of these tumors are located in the middle of the brainstem making them inoperable and difficult to treat.
Choroid plexus tumors: These tumors develop in the choroid plexus cells in the ventricles of the brain. These cells are responsible for producing cerebrospinal fluid which cushions the brain and spinal cord. This type of tumor may result in hydrocephalus, or a buildup of cerebrospinal fluid.
Craniopharyngiomas: These benign tumors are located near the pituitary gland, responsible for producing hormones controlling other glands in the body. Tumors in this area may cause hormonal imbalances. Surgical removal is usually the preferred method of treatment.
Germ cell tumors: These tumors arise from germ cells (eggs or sperm) during development and may be benign or malignant.
Medulloblastomas: These brain tumors account for approximately 15% of pediatric brain tumors and usually develop between the ages of 4-9 and disproportionately more in males. These tumors are malignant and may metastasize to the spinal cord. Treatment typically is a combination of surgery and other methods.
Optic nerve gliomas: This is a glioma found in or surrounding the optic nerves. These tumors are more frequently discovered in children with neurofibromatosis, a genetic disorder or the nervous system. Optic nerve gliomas cause impaired vision and hormonal problems due to the location. Treatment can be difficult due to sensitive surrounding brain structures.
When planning for a pediatric brain tumor surgery, the neurosurgery team needs to know the tumor:
- Type: Histological examination determines the tumor cell type. Cell type informs the physicians of the likelihood of growth and metastasis.
- Location: Neuroimaging, usually through a form of MRI, provides detailed information on the exact tumor location. Tumor location partially determines if surgery is a viable option as certain brain areas are inoperable. Surgical approach is also partially determined by tumor location.
- Grade: Tumor grade refers to the level of cell abnormality and aggressiveness of the tumor.
Treatment for Pediatric Brain Tumors
Surgery: The majority of childhood brain tumors necessitate surgical removal. The initial surgery may entail complete removal or partial removal to alleviate elevated intracranial pressure. Surgical removal may be the only treatment necessary for low-grade tumors.
Follow-Up Care: Future treatment and recovery process of the child after surgery is largely dependent on the type, location, and size of the tumor as well as the timing of diagnosis and treatment. The prognosis is highly variable and case dependent. Regular post-operative and follow-up visits are needed to monitor neurological status, side effects, progression, or recurrence.
Radiation Therapy consists of focusing high doses of radiation directly on the tumor itself and surrounding tissue. In infants and young children, radiation is used sparingly as the brain is still in critical development. However certain tumors, such as medulloblastomas, may require radiation to the entire brain or spinal cord.
Chemotherapy utilizes anti-cancer chemicals systemically for high-grade, aggressive tumors. Chemotherapy may be administered in the form of pills (orally), intravenously (vein), or injection (cerebrospinal fluid or brain cavity after surgical removal).
Head Trauma
 The term head trauma is exceedingly broad and may range from minor symptoms to severe and life-threatening complications. Traumatic head injury may cause concussions, contusions, lacerations, or brain damage. This may clinically manifest as loss of consciousness (from second to hours) or, in severe cases, leads to coma. Head trauma may also result in further complications such as hemorrhages in the epidural, subdural, subarachnoid, or intracerebral areas.
The term head trauma is exceedingly broad and may range from minor symptoms to severe and life-threatening complications. Traumatic head injury may cause concussions, contusions, lacerations, or brain damage. This may clinically manifest as loss of consciousness (from second to hours) or, in severe cases, leads to coma. Head trauma may also result in further complications such as hemorrhages in the epidural, subdural, subarachnoid, or intracerebral areas.
Well known that visual impairment is frequently associated with head trauma and typically occurs immediately thereafter. Vision lost due to significant head trauma is sometimes referred to as cortical visual impairment (CVI). CVI does not exclusively occur due to head trauma, but is a general term describing vision loss due to impaired neurological signaling along the visual pathway in the brain. The long-term effects of CVI vary significantly ” ranging from total recovery of the eyesight within days to permanent vision loss. Understandably, more severe cases of head trauma are associated with a worse prognosis when compared to minor head trauma cases. Patients afflicted with severe head trauma frequently have long-term visual sequelae and permanent neurological deficits. These cases typically are evident through neuroimaging, revealing skull fractures, intracranial hemorrhages, or cerebral injury.
While after a traumatic event certain symptoms do typically improve quickly ” such as headaches, irritability, blindness, light sensitivity, disorientation ” other symptoms such as cognitive deficits and visual field defects may persist due to posttraumatic injury and /or atrophy of brain tissue. Common complications arising after head trauma include hydrocephalus, cerebral edema, and hemorrhages. Cerebral dysfunction due to head trauma may also result in concussions, epilepsy, brain tissue edema, or ischemia.
Common visual symptoms of traumatic brain damage may include blurred central vision, missing peripheral vision, light sensitivity, spatial orientation difficulty, reduced peripheral awareness, double vision, or fine flickering of vision resembling TV static (visual snow). Sometimes neuroimaging results are negative as certain neurological disturbances, like concussions, are functional rather than structural in nature.
Young children often are unable to recognize vision loss or vocalize visual disturbances, however these children may exhibit signs of irritability, uncooperativeness, restlessness, confusion, disorientation, lack of coordination, headaches, or drowsiness. Therefore, trauma induced CVI should be suspected in children exhibiting such behavior as this may be a psychological reaction to either the brain trauma or visual disturbances. Visual disturbances may arise due to damage of the posterior visual pathway likely from the coup or contrecoup forces from the trauma. Transient blindness potentially occurs due to local cerebral vasospasm similar to the pathophysiology of migraines. Similarly, visual snow symptoms have been associated with migraines and may have a vascular etiology. Persons with a family history of migraines seem to be at increased risk for transient vision loss following a traumatic brain event. Certain persons may be predisposed to these vascular disturbances following head trauma, as evidenced by certain people repeatedly having such transient vision loss following striking a soccer ball with their head on multiple occasions (aka œfootballer's migraine). This and other cases suggests certain persons, likely with a family history of migraines, are at increased risk of visual disturbances due to cerebral blood flow alterations following even minor head trauma.
Sadly, CVI may also occur due to child abuse and shaken baby syndrome. Such trauma may induce intracranial hemorrhages, concussive cerebral injury, subdural hematomas, or retinal hemorrhages from a young age. Such diagnoses warrant consideration when these neurological signs are present along with signs of physical trauma.
Metabolic disturbances
Significant metabolic disturbances may lead to not only optic neuropathy, but also cortical visual impairment (CVI). These disturbances could be due to extreme hypoglycemia, poisoning (carbon monoxide, lead, nitric oxide), uremia, hemodialysis, alcohol, or cocaine exposure. CVI is also associated with neurodegenerative disorders such as metabolic encephalopathy, lactic acidosis, mitochondrial encephalopathy, Leighs disease, X-linked adrenoleukodystrophy, and strokes.
Meningitis, Encephalitis, and Sepsis
A small number of CVI cases are caused by pathogens. Roughly 5% of CVI results from bacterial meningitis during infancy. Meningitis has well-known neurological complications such as cognitive deficits, seizures, hemiplegia, and quadriplegia. However, meningitis is also associated with significant visual impairment, visual field defects, and visual hallucinations. The most likely organisms are haemophilus influenzae, pneumococci, and streptococci. Meningitis induced CVI occurs within 1 week for 50% of cases and within 1 month for essentially all cases. For unknown reasons, haemophilus influenzae seems to target the occipital cortex, largely responsible for vision. Post-meningitic CVI may be a consequence of hydrocephalus, ischemia, venous sinus thrombosis, or thrombophlebitis. As with CVI in general, post-meningitic CVI may have partial recovery, complete recovery, or permanent vision loss.
Viral encephalitis caused by herpes simplex virus is associated with severe CVI with the vast majority (~80%) of these cases being caused by Type 2 herpes simplex. These cases usually involve severe brain damage from necrotizing encephalopathy, widespread neurological disease, and demyelination causing diffuse white matter damage. Severe cortical damage and optic atrophy are both frequently associated with neonatal herpes simplex virus, leading to profound vision loss.
Hydrocephalus, Ventricular Shunt Failure
Hydrocephalus, or buildup of excessive cerebrospinal fluid in the ventricles of the brain, exerts pressure on the brain which may cause visual impairment. Hydrocephalus may arise from multiple disease processes including meningitis, space occupying lesions, enlarged brain ventricles, brain malformation. Hydrocephalic brain damage and dysfunction is multifaceted.
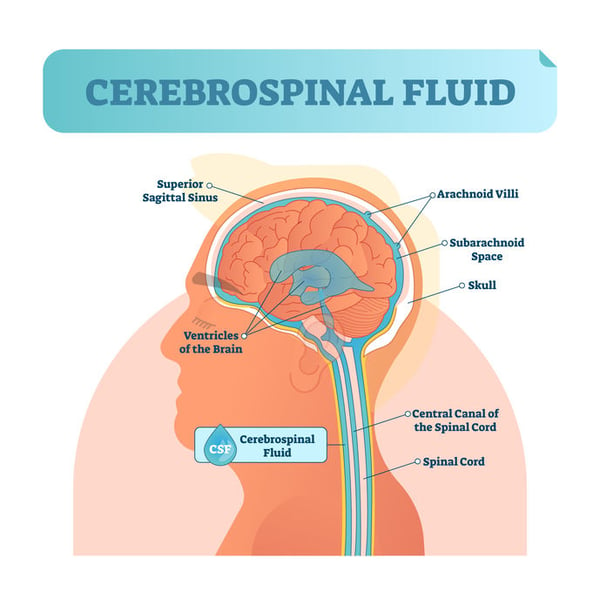
Hydrocephalus may cause axonal dysfunction through mechanical stretching or compression of tissue as well as from ischemia due to impaired blood flow, especially in white matter. Inflammation and oxidative stress may further deteriorate axons. Extracellular fluid dynamics might also be altered due to excess cerebrospinal fluid.
Visual impairment from hydrocephalus may present as visual field defects, the most common being homonymous hemianopia, or loss of vision to the right or to the left. Hydrocephalus may cause damage to either the anterior or posterior visual pathway. Anterior visual pathway damage may occur from papilledema-induced optic atrophy, compression of the optic chiasm from the third ventricle, compression of the optic tracts, developmental abnormalities, or vascular deficiencies causing ischemia. Posterior visual pathway damage may result from compression of the posterior cerebral arteries potentially causing laminar necrosis in the visual cortex. Although hydrocephalus may cause a variety of neurological problems, the visual system is commonly affected. In children vision problems manifest as reduced visual acuity, visual field defects, strabismus, eye movement disorders, and impaired orientation and depth perception.
Treatment of hydrocephalus, oftentimes using a shunt, may partially or completely resolve visual field defects. If hydrocephalus reoccurs after a shunt procedure, a shunt revision may be necessary. Vision disturbance may be the first or only sign of a shunt failure. Vision generally improves over the course of several years, although may improve as quickly as a few days.

 We draw your attention to the fact that the
We draw your attention to the fact that the
.jpg?width=500&name=Fotos%20-%201%20von%201%20(1).jpg)

 An initial eye examination by eye care providers reveals clues about the underlying cause of the visual disturbance. Below we have collected some helpful signs which suggest potential ocular and/or visual abnormalities. Some of them are: congenital nystagmus, pupillary response to light, nearsightedness or farsightedness, photophobia (light sensitivity), “oculodigital sign”, overlooking, excess head and eye movements
An initial eye examination by eye care providers reveals clues about the underlying cause of the visual disturbance. Below we have collected some helpful signs which suggest potential ocular and/or visual abnormalities. Some of them are: congenital nystagmus, pupillary response to light, nearsightedness or farsightedness, photophobia (light sensitivity), “oculodigital sign”, overlooking, excess head and eye movements
 Congenital stationary night blindness (CSNB) is a collection of non-progressive inherited retinal diseases (IRD) characterized by impaired night vision, reduced central and peripheral vision, nystagmus, nearsightedness (myopia), and strabismus (eye turn). CSNB may be classified by both retinal appearance and electrophysiology findings.
Congenital stationary night blindness (CSNB) is a collection of non-progressive inherited retinal diseases (IRD) characterized by impaired night vision, reduced central and peripheral vision, nystagmus, nearsightedness (myopia), and strabismus (eye turn). CSNB may be classified by both retinal appearance and electrophysiology findings. CSNB has multiple inheritance patterns including autosomal dominant, autosomal recessive, and X-linked. Numerous pathological variants also exist for CSNB. Pathological variants are genetic alterations which predispose an individual to a particular disease or condition through altering normal protein function or protein interactions. Pathological variants for CSNB include CABP4, GNB3, GPR179, GRM6, LRIT3, SAG, SLC24A1, TRPM1, GNAT1, PDE6B, RHO, CACNA1F, and NYX. As with all inherited retinal diseases, genetic testing is beneficial in confirming the diagnosis, determining the specific gene(s) at play, ascertaining the carrier status of the child and family members, and deciding best treatment approaches.
CSNB has multiple inheritance patterns including autosomal dominant, autosomal recessive, and X-linked. Numerous pathological variants also exist for CSNB. Pathological variants are genetic alterations which predispose an individual to a particular disease or condition through altering normal protein function or protein interactions. Pathological variants for CSNB include CABP4, GNB3, GPR179, GRM6, LRIT3, SAG, SLC24A1, TRPM1, GNAT1, PDE6B, RHO, CACNA1F, and NYX. As with all inherited retinal diseases, genetic testing is beneficial in confirming the diagnosis, determining the specific gene(s) at play, ascertaining the carrier status of the child and family members, and deciding best treatment approaches. 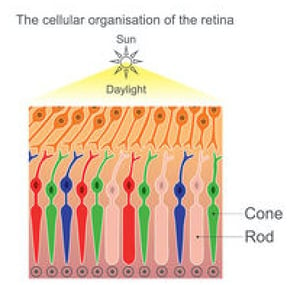 Achromatopsia, or rod monochromatism, is characterized by a functional absence of all three cone photoreceptor subtypes from birth. One major function of cone photoreceptors is color vision distinction, explaining why achromatopsia is sometimes referred to as total color blindness. Worldwide this condition affects approximately 1/30,000 persons.
Achromatopsia, or rod monochromatism, is characterized by a functional absence of all three cone photoreceptor subtypes from birth. One major function of cone photoreceptors is color vision distinction, explaining why achromatopsia is sometimes referred to as total color blindness. Worldwide this condition affects approximately 1/30,000 persons. 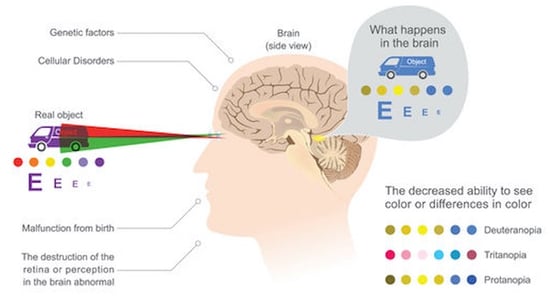 The deficient cone photoreceptor signaling in congenital achromatopsia is present at birth meaning the visual cortex never receives normal visual input. For this reason the neurological wiring, organization, and processing of the visual cortex never develops properly. The term for this abnormal input and processing leading to vision loss is amblyopia. Although best treated in young children, some evidence exists that amblyopia may be partially treatable in adults depending on the level of plasticity of the visual cortex, which is controversial. At least one study suggests that visual function may be improved in adult patients with achromatopsia. Treatment aims to improve both the cone photoreceptor signaling deficient in achromatopsia as well as the amblyopia caused by the achromatopsia.
The deficient cone photoreceptor signaling in congenital achromatopsia is present at birth meaning the visual cortex never receives normal visual input. For this reason the neurological wiring, organization, and processing of the visual cortex never develops properly. The term for this abnormal input and processing leading to vision loss is amblyopia. Although best treated in young children, some evidence exists that amblyopia may be partially treatable in adults depending on the level of plasticity of the visual cortex, which is controversial. At least one study suggests that visual function may be improved in adult patients with achromatopsia. Treatment aims to improve both the cone photoreceptor signaling deficient in achromatopsia as well as the amblyopia caused by the achromatopsia.  The Latin root word of albinism is “albus”, meaning white. Albinism is a congenital disorder characterized by decreases in melanin of not only the skin and hair, but also the pigmentation in the eye. Albinism of the eye only is known as ocular albinism (OA) while albinism of the eyes, skin, and hair is referred to as oculocutaneous albinism (OCA). Albinism occurs due to deficiencies in proteins or enzymes which contribute to melanin synthesis lead to markedly reduced or even absent pigmentation.
The Latin root word of albinism is “albus”, meaning white. Albinism is a congenital disorder characterized by decreases in melanin of not only the skin and hair, but also the pigmentation in the eye. Albinism of the eye only is known as ocular albinism (OA) while albinism of the eyes, skin, and hair is referred to as oculocutaneous albinism (OCA). Albinism occurs due to deficiencies in proteins or enzymes which contribute to melanin synthesis lead to markedly reduced or even absent pigmentation.  Interestingly, melanin and pigmented cells appear to play a role in the development of the visual pathway from the eye to the brain, specifically at the optic nerve and chiasmal decussation behind the optic nerve. The reduction or absence of this pigment is believed to result in a misrouting of optic nerve axons at the optic chiasm. This miswiring along the visual pathway affects visual input and processing and results in amblyopia. Normally temporal optic nerve axons do not cross at the optic chiasm, but rather, remain on the original side of the brain. The temporal optic nerve axons in patients with OA, however, irregularly cross over to the other side of the brain. This abnormal number of axons decussating (crossing) the optic chiasm results is detectable via visual evoked potentials.
Interestingly, melanin and pigmented cells appear to play a role in the development of the visual pathway from the eye to the brain, specifically at the optic nerve and chiasmal decussation behind the optic nerve. The reduction or absence of this pigment is believed to result in a misrouting of optic nerve axons at the optic chiasm. This miswiring along the visual pathway affects visual input and processing and results in amblyopia. Normally temporal optic nerve axons do not cross at the optic chiasm, but rather, remain on the original side of the brain. The temporal optic nerve axons in patients with OA, however, irregularly cross over to the other side of the brain. This abnormal number of axons decussating (crossing) the optic chiasm results is detectable via visual evoked potentials.  Childhood glaucoma — also termed pediatric glaucoma, infantile glaucoma, or congenital glaucoma — is responsible for approximately 7% of visual impairment in the pediatric population worldwide.
Childhood glaucoma — also termed pediatric glaucoma, infantile glaucoma, or congenital glaucoma — is responsible for approximately 7% of visual impairment in the pediatric population worldwide. These developmental defects may occur due to genetics, other eye problems, or from systemic disease. Improper drainage of aqueous fluid in the front of the eye leads to a rise in intraocular pressure which may injure the optic nerve due to mechanical pressure or blood flow impairment.
These developmental defects may occur due to genetics, other eye problems, or from systemic disease. Improper drainage of aqueous fluid in the front of the eye leads to a rise in intraocular pressure which may injure the optic nerve due to mechanical pressure or blood flow impairment.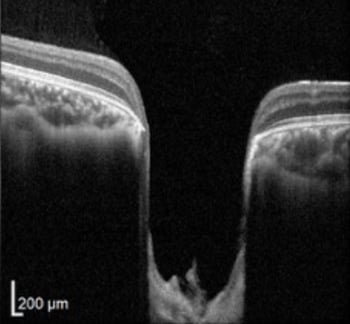
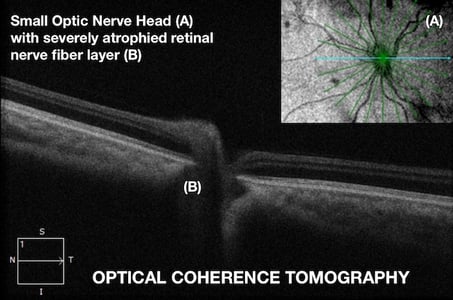 The presence of optic nerve hypoplasia in both eyes, rather than only one eye, increases the risk of developmental delays. Other ocular abnormalities may include strabismus (usually esotropia), nystagmus, microphthalmus, and coloboma. The optic nerve head may display signs of hypoplasia such as an underdeveloped appearance, a double ring sign, or pallor. The degree of visual impairment is highly variable, from near normal to light perception only.
The presence of optic nerve hypoplasia in both eyes, rather than only one eye, increases the risk of developmental delays. Other ocular abnormalities may include strabismus (usually esotropia), nystagmus, microphthalmus, and coloboma. The optic nerve head may display signs of hypoplasia such as an underdeveloped appearance, a double ring sign, or pallor. The degree of visual impairment is highly variable, from near normal to light perception only. Better understanding of the development of the pituitary gland and the forebrain has led to the discovery of two genes responsible for septo-optic dysplasia, HESX1 and SOX2. However, the role of genetics in SOD is currently limited as genetic abnormalities are detected in less than 1% of cases. This has stirred debate over other causative genes or pathological sources of SOD. A vascular etiology is one proposed mechanism for SOD development as is certain environmental factors such as tobacco, alcohol, or other drugs. More research is needed to fully understand the mechanisms and genetic pathological variants responsible for SOD. Presently most cases are believed to occur sporadically with an absence of family history.
Better understanding of the development of the pituitary gland and the forebrain has led to the discovery of two genes responsible for septo-optic dysplasia, HESX1 and SOX2. However, the role of genetics in SOD is currently limited as genetic abnormalities are detected in less than 1% of cases. This has stirred debate over other causative genes or pathological sources of SOD. A vascular etiology is one proposed mechanism for SOD development as is certain environmental factors such as tobacco, alcohol, or other drugs. More research is needed to fully understand the mechanisms and genetic pathological variants responsible for SOD. Presently most cases are believed to occur sporadically with an absence of family history. 
 This condition is occasionally more broadly referred to as cerebral, rather than cortical in nature, as reduced vision may occur due to abnormalities in the subcortical optic radiations or occipital cortex. The incidence of CVI has been steadily rising over the past few decades and currently around 160 cases per 100,000 individuals. Worldwide, CVI is a major contributor to childhood vision loss. Visual function is diminished in CVI due to reduced or absent signaling from the eye to the brain. The degree of visual impairment ranges from mild to complete vision loss, called cortical blindness.
This condition is occasionally more broadly referred to as cerebral, rather than cortical in nature, as reduced vision may occur due to abnormalities in the subcortical optic radiations or occipital cortex. The incidence of CVI has been steadily rising over the past few decades and currently around 160 cases per 100,000 individuals. Worldwide, CVI is a major contributor to childhood vision loss. Visual function is diminished in CVI due to reduced or absent signaling from the eye to the brain. The degree of visual impairment ranges from mild to complete vision loss, called cortical blindness. Solid brain tumors develop in approximately 30 out of 1,000,000 children and have a 10 year survival rate of just under 60%. Diagnosis is made through both clinical history and neuroradiological imaging and confirmed through histology. Despite the rare diagnosis and survival rate, brain tumors are the leading cause of cancer deaths in children. These tumors can cause significant visual impairments if the tumor is located in areas of the visual system or if the tumor leads to hydrocephalus (elevated intracranial pressure). Such visual impairments may negatively affect a childs everyday activities, development, education, and vocation if not treated effectively or in a timely manner.
Solid brain tumors develop in approximately 30 out of 1,000,000 children and have a 10 year survival rate of just under 60%. Diagnosis is made through both clinical history and neuroradiological imaging and confirmed through histology. Despite the rare diagnosis and survival rate, brain tumors are the leading cause of cancer deaths in children. These tumors can cause significant visual impairments if the tumor is located in areas of the visual system or if the tumor leads to hydrocephalus (elevated intracranial pressure). Such visual impairments may negatively affect a childs everyday activities, development, education, and vocation if not treated effectively or in a timely manner.  Neurological examination and neuroimaging are the first steps taken to confirm or deny the presence of an intracranial process as a tumor. Multiple scan types may be necessary to confirm the presence or absence of a tumor depending on the size, location, and type of tumor. Common neuroimaging methods include:
Neurological examination and neuroimaging are the first steps taken to confirm or deny the presence of an intracranial process as a tumor. Multiple scan types may be necessary to confirm the presence or absence of a tumor depending on the size, location, and type of tumor. Common neuroimaging methods include: The term head trauma is exceedingly broad and may range from minor symptoms to severe and life-threatening complications. Traumatic head injury may cause concussions, contusions, lacerations, or brain damage. This may clinically manifest as loss of consciousness (from second to hours) or, in severe cases, leads to coma. Head trauma may also result in further complications such as hemorrhages in the epidural, subdural, subarachnoid, or intracerebral areas.
The term head trauma is exceedingly broad and may range from minor symptoms to severe and life-threatening complications. Traumatic head injury may cause concussions, contusions, lacerations, or brain damage. This may clinically manifest as loss of consciousness (from second to hours) or, in severe cases, leads to coma. Head trauma may also result in further complications such as hemorrhages in the epidural, subdural, subarachnoid, or intracerebral areas. 

 Note: The information given in this blog are the opinions of the authors and for reader familiarization purposes only. This blog is not intended as a substitute for professional medical advice. Also, the information provided does not replace or abolish any official or legal terms for diagnosis, treatment, and management. Authors are not liable for any undesirable consequences or effects related to the information provided in the blog.
Note: The information given in this blog are the opinions of the authors and for reader familiarization purposes only. This blog is not intended as a substitute for professional medical advice. Also, the information provided does not replace or abolish any official or legal terms for diagnosis, treatment, and management. Authors are not liable for any undesirable consequences or effects related to the information provided in the blog.