Diagnostic Testing
When considering NAION as a diagnosis, a meticulous medical and social history is needed in addition to a thorough eye examination. Questioning pertaining to sleep apnea, giant cell arteritis (GCA), and erectile dysfunction drugs is warranted.
 Although NAION may present with findings similar to that of GCA, a classic presentation of NAION without GCA signs or symptoms may not warrant the usual GCA testing of a complete blood count (CBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). However, if there is any doubt then CBC, ESR, and CRP testing is indicated to rule out GCA. Patients should always be evaluated by a primary-care physician for potential modifiable or treatable risk factors including smoking, sleep apnea, hypertension, diabetes, hyperlipidemia, and other vascular disorders. Neuroimaging is only performed in cases diverging from a typical NAION course such as prolonged optic disc edema, progressing vision loss, or unusual pain. Except in cases of family history or personal clinical evidence, there is no proven value of additional vasculopathic or prothrombotic diagnostic testing (ex. carotid studies, hypercoagulability testing), however homocysteine levels may be tested in patients under the age of 50. Patients with significant hyperhomocysteinemia may be recommended supplementation of vitamins B6, B12, and folic acid and regular monitoring for systemic vasculopathies.
Although NAION may present with findings similar to that of GCA, a classic presentation of NAION without GCA signs or symptoms may not warrant the usual GCA testing of a complete blood count (CBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). However, if there is any doubt then CBC, ESR, and CRP testing is indicated to rule out GCA. Patients should always be evaluated by a primary-care physician for potential modifiable or treatable risk factors including smoking, sleep apnea, hypertension, diabetes, hyperlipidemia, and other vascular disorders. Neuroimaging is only performed in cases diverging from a typical NAION course such as prolonged optic disc edema, progressing vision loss, or unusual pain. Except in cases of family history or personal clinical evidence, there is no proven value of additional vasculopathic or prothrombotic diagnostic testing (ex. carotid studies, hypercoagulability testing), however homocysteine levels may be tested in patients under the age of 50. Patients with significant hyperhomocysteinemia may be recommended supplementation of vitamins B6, B12, and folic acid and regular monitoring for systemic vasculopathies.
Differential Diagnosis
Differential diagnoses for NAION primarily include other anterior optic neuropathies or orbital lesions. Differentials include:
- Anterior optic neuritis
- Demyelinating
- Sarcoidosis
- Idiopathic
- Other
- Infiltrative optic neuropathy
- Leukemia
- Lymphoma
- Sarcoidosis
- Other
- Orbital lesion causing anterior compressive optic neuropathy
NAION vs Optic Neuritis
Optic neuritis may closely resemble NAION in regards to vision loss, visual field defects, optic disc appearance, and symptom onset; however key differences include age, pain with eye movement, and type of disc edema. Optic neuritis patients are generally younger, report pain with eye movement, and have diffuse disc edema without hemorrhages. Optic neuritis patients may also have retrobulbar optic nerve swelling in which case the optic nerve head appears normal. NAION disc edema is more likely to be altitudinal or segmental in nature and have disc pallor, vessel attenuation, and hemorrhages. Orbital lesions may result in compressive optic neuropathy and present as unilateral optic nerve head edema with slow, but progressive vision loss. However, orbital lesions also may cause lid proptosis, eye movement abnormalities, lid abnormalities, and non-resolving optic disc swelling. Infiltrative optic neuropathies, similarly, may demonstrate persistent optic disc edema and cause slowly progressing vision loss.
Arteritic IONs
Many vasculopathies may trigger anterior or posterior ischemic optic neuropathy, the most common of which being giant-cell arteritis (GCA). Anterior ischemic optic neuropathy due to GCA is called arteritic anterior ischemic optic neuropathy (AAION). GCA has an annual incidence of 18 people per 100,000 in persons over the age of 50. Serious vision loss occurs in approximately 20% of these cases. The most frequent ocular manifestation of GCA is AION however both AION and PION may produce calamitous vision loss and therefore should be treated as an emergency. Although the clinical presentation of GCA and NAION are similar, systemic symptoms of GCA may be differentiating factors. Systemic symptoms of scalp tenderness, severe head pain, jaw pain, and general malaise oftentimes precede vision loss by weeks to months. Vision loss is also generally preceded by visual symptoms of transient blurriness or diplopia. However, known as occult GCA, one quarter of GCA patients present with isolated ischemic optic neuropathy and no systemic symptoms.
AAION induced vision loss is typically more serious than NAION with over half of AAION patients being unable to count fingers as compared to approximately a quarter of patients with NAION. AAION is considered an ocular emergency because untreated AAION involves the fellow eye in well over 50% of cases within days to week.
Optic nerve pallor is a differentiating factor between AAION and NAION as the affected optic nerve often presents immediately with pallor in AAION while pallor is delayed in NAION. While a “disc at risk” is suggestive of NAION, AAION may occur with any cup-to-disc ratio. Additionally, unlike NAION, GCA may also cause ischemia of the retina or choroid.
Erythrocyte sedimentation rate (ESR), C-reactive protein level (CRP), and complete blood count (CBC) should be performed on any patient over the age of 50 with ION who are being considered for GCA because contralateral vision loss is rapid and common. The results of the combination of these diagnostic tests is highly predictive for GCA (sensitivity of 97%). When these tests confirm GCA, the treatment generally entails high doses of intravenous steroids.
Perioperative ION
Various non-ophthalmic surgeries may cause severe bilateral vision loss by inducing anterior and posterior ION. Coronary artery bypass grafting and prolonged spinal-fusion surgery are the two surgeries most likely to provoke ION. However, it is worth noting that the reported incidence of this complication is less than 0.3%. For reasons that are unclear and likely multifactorial, cardiac surgery is more associated with AION while spinal surgery is more associated with PION. While the mechanism(s) are poorly understood, ultimately, axonal injury arises from either a vascular or compressive source.
Potential pathways for axonal damage include:
- Decreased perfusion pressure
- Reduced oxygen delivery
- Elevated venous pressure
- Direct compressive axonal injury
Proposed mechanisms contributing to these pathways include:
- Tissue hypoxia
- Anemia
- Blood loss
- Hypotension
- Increased interstitial fluid in the optic nerve
- Increased interstitial fluid in the orbit
- Vasoconstricting agents used preoperatively or postoperatively
- Increased intraocular pressure (only anterior ION)
- Predisposing vascular or thrombotic risk factors
- Anatomical or physiological predisposing factors (ex. small cup-to-disc ratio or vascular watershed regions in the posterior optic nerve)
Risk factors for development of ION in patients undergoing spinal-fusion surgery in a prone position include:
- Male
- Obesity
- Total time under anesthesia
- Total blood loss
NAION and Medications
Certain medications have been linked with NAION development to treat malignant tumors (ex. melanomas, leukemia, and lymphomas) and chronic hepatitis C, has been associated with bilateral, sequential NAION. The severity and prognosis vary, but some patients demonstrate recovery after discontinuing such medications. Potential mechanisms include systemic hypotension or deposition of immune complexes within the optic disc vasculature.
Three drugs prescribed to treat erectile dysfunction (ED) have been temporally associated with NAION onset, likely by producing systemic hypotension. Therapeutic doses of these ED medications may decrease systemic blood pressure over 10 mmHg. Each reported case connecting these medications with NAION occured in patients with “discs-at-risk”. The combination of these structurally crowded discs with augmented nocturnal hypotension is believed to have triggered an ischemic event. Despite the fact that the number of cases connecting NAION with ED drugs is modest, male NAION patients should be asked about ED medication usage. It is also pragmatic for patients being administered ED drugs to have a complete eye examination to assess for structural crowding of the optic discs.
Commonly used medicine to treat cardiac arrhythmias has been linked with an anterior optic neuropathy resembling NAION. It is worthwhile to mention that patients using this drugs generally already have vasculopathic risk factors predisposing them to NAION development. Several studies have revealed that NAION typically involves cases that are bilateral, have an insidious onset, involve general visual field loss (rather than altitudinal), and which have optic nerve edema persisting for months (rather than weeks).

 Patients generally present with a painless and sudden onset of vision loss in one eye accompanied by peripheral vision disturbances, noticed in the morning by over 66% of patients. Although eye pain is rare in NAION, approximately 8-12% of patients report periocular eye pain which may initially make AION difficult to discern from optic neuritis. However in NAION, unlike optic neuritis, patients never present with pain during eye movements.
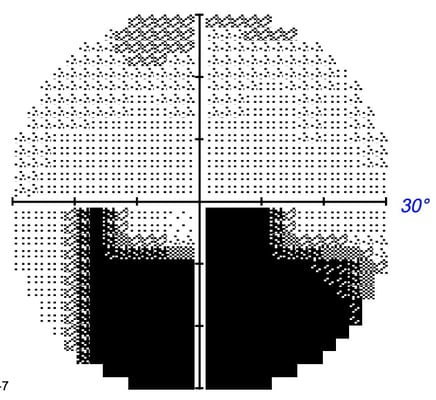
Patients generally present with a painless and sudden onset of vision loss in one eye accompanied by peripheral vision disturbances, noticed in the morning by over 66% of patients. Although eye pain is rare in NAION, approximately 8-12% of patients report periocular eye pain which may initially make AION difficult to discern from optic neuritis. However in NAION, unlike optic neuritis, patients never present with pain during eye movements. 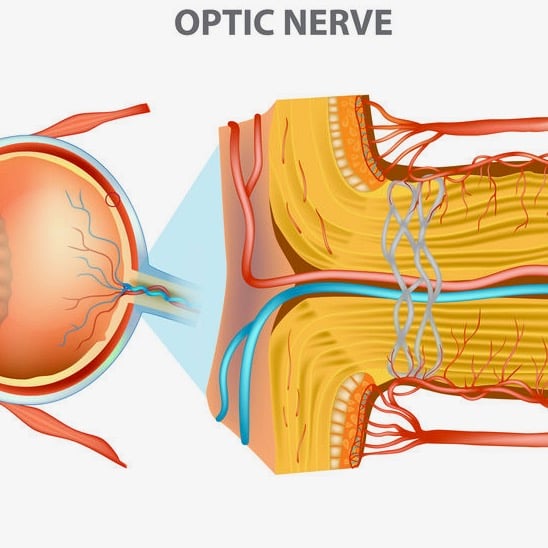 This is evident during examination of the fundus as optic disc edema, oftentimes with optic disc hyperemia and peripapillary splinter-shaped hemorrhages. This disc edema usually resolves in 4-6 weeks with sectoral or diffuse optic disc pallor potentially being sequelae. Although uncommon, cotton wool spots (evidence of retinal ischemia) may also be present. AION incidents are far more common in persons with a physiologically small cup-to-disc ratio, the area of the optic nerve where axons enter the eye. While a normal ratio is approximately 0.3, a ratio less than 0.25 is considered a “crowded disc” or a “disc at risk” for an AION episode. Recurrence of AION episodes in the affected eye are rare, estimated to be only 3-8%. However, the likelihood of involvement of the fellow eye over the following 5 years ranges from 15-24%.
This is evident during examination of the fundus as optic disc edema, oftentimes with optic disc hyperemia and peripapillary splinter-shaped hemorrhages. This disc edema usually resolves in 4-6 weeks with sectoral or diffuse optic disc pallor potentially being sequelae. Although uncommon, cotton wool spots (evidence of retinal ischemia) may also be present. AION incidents are far more common in persons with a physiologically small cup-to-disc ratio, the area of the optic nerve where axons enter the eye. While a normal ratio is approximately 0.3, a ratio less than 0.25 is considered a “crowded disc” or a “disc at risk” for an AION episode. Recurrence of AION episodes in the affected eye are rare, estimated to be only 3-8%. However, the likelihood of involvement of the fellow eye over the following 5 years ranges from 15-24%.  47-49% of NAION patients have hypertension while 24-26% have diabetes. Other potential, but unconfirmed risk factors include arteriosclerosis, lipohyalinosis, smoking, vascular autoregulation failures, sleep apnea, vasospasm, anemia, nocturnal hypotension, and generalized hypoperfusion.
47-49% of NAION patients have hypertension while 24-26% have diabetes. Other potential, but unconfirmed risk factors include arteriosclerosis, lipohyalinosis, smoking, vascular autoregulation failures, sleep apnea, vasospasm, anemia, nocturnal hypotension, and generalized hypoperfusion. Patients previously diagnosed with optic nerve circulation disorders or systemic hypotension have a heightened risk of NAION development. One major cause for concern is nocturnal hypotension secondary to antihypertensive medicinal therapy -- medications frequently used by individuals in age range for NAION development. Aggressive antihypertensive treatment or medications administered in the evenings may provoke an NAION episode by exagerrating normal systemic nocturnal hypotension. In hypertensive patients with NAION, studies indicate that those patients with visual field loss demonstrate significantly lower blood pressure than those patients with no visual field loss. This research suggests that certain individuals may be at increased risk of both NAION development and NAION progression due to exaggerated nocturnal hypotension, especially in cases of aggressive hypertension treatment.
Patients previously diagnosed with optic nerve circulation disorders or systemic hypotension have a heightened risk of NAION development. One major cause for concern is nocturnal hypotension secondary to antihypertensive medicinal therapy -- medications frequently used by individuals in age range for NAION development. Aggressive antihypertensive treatment or medications administered in the evenings may provoke an NAION episode by exagerrating normal systemic nocturnal hypotension. In hypertensive patients with NAION, studies indicate that those patients with visual field loss demonstrate significantly lower blood pressure than those patients with no visual field loss. This research suggests that certain individuals may be at increased risk of both NAION development and NAION progression due to exaggerated nocturnal hypotension, especially in cases of aggressive hypertension treatment.  Although the precise mechanism is unclear, “discs at risk” or crowded optic discs are more susceptible to ischemia possibly due to mechanical stress of the local circulatory system. Sleep apnea commonly causes recurrent nocturnal ischemic episodes and may be associated with NAION.
Although the precise mechanism is unclear, “discs at risk” or crowded optic discs are more susceptible to ischemia possibly due to mechanical stress of the local circulatory system. Sleep apnea commonly causes recurrent nocturnal ischemic episodes and may be associated with NAION. 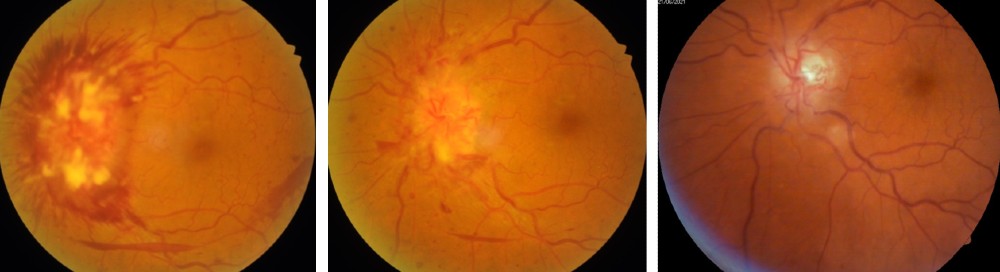 Secondly, this edema impairs the transportation of essential nutrients and neurotransmitters to retinal ganglion cells thus damaging the retinal ganglion cell layer by means of oxidative stress, excitotoxicity, metabolic imbalances, mitochondrial failure, or apoptosis.
Secondly, this edema impairs the transportation of essential nutrients and neurotransmitters to retinal ganglion cells thus damaging the retinal ganglion cell layer by means of oxidative stress, excitotoxicity, metabolic imbalances, mitochondrial failure, or apoptosis. 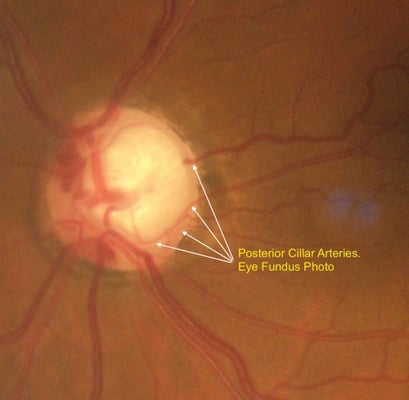 The ophthalmic artery branch of the internal carotid artery is responsible for the vast majority of the blood supply to the optic nerve. The inner retinal layers receive blood flow from a branch of the ophthalmic artery called the central retinal artery, which enters the optic nerve roughly one centimeter behind the globe. However, the outer retinal layers depend on the choroidal arteries, which stem from the posterior ciliary arteries, for blood supply.
The ophthalmic artery branch of the internal carotid artery is responsible for the vast majority of the blood supply to the optic nerve. The inner retinal layers receive blood flow from a branch of the ophthalmic artery called the central retinal artery, which enters the optic nerve roughly one centimeter behind the globe. However, the outer retinal layers depend on the choroidal arteries, which stem from the posterior ciliary arteries, for blood supply. 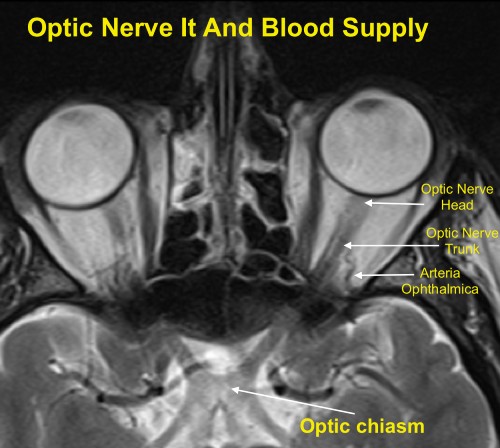 Ischemic optic neuropathy (ION) may present during hypoperfusion of the ophthalmic artery or its subsequent branches. The clinical signs and patient symptoms vary depending on the region of the optic nerve in which the ischemia occurs. The optic disc appears normal in cases of posterior optic nerve ischemia while the optic disc is edematous in cases of anterior optic nerve ischemia.
Ischemic optic neuropathy (ION) may present during hypoperfusion of the ophthalmic artery or its subsequent branches. The clinical signs and patient symptoms vary depending on the region of the optic nerve in which the ischemia occurs. The optic disc appears normal in cases of posterior optic nerve ischemia while the optic disc is edematous in cases of anterior optic nerve ischemia. 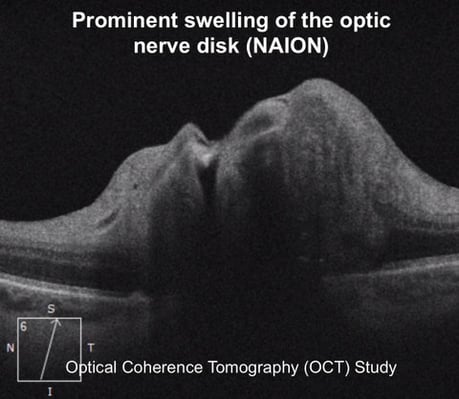 The optic disc swelling accompanying NAION may be diffuse or segmental with or without hyperemia. Pallor may occur after resolution of the disc edema or, rarely, during the disc edema. Peripapillary splinter hemorrhages are commonly observed while cotton wool spots and macular hard exudates are seldom found. In more advanced cases, retinal arterioles may be narrowed. The contralateral eye (i.e. the non-affected eye) usually reveals a small cup-to-disc ratio or a “disc at risk.”
The optic disc swelling accompanying NAION may be diffuse or segmental with or without hyperemia. Pallor may occur after resolution of the disc edema or, rarely, during the disc edema. Peripapillary splinter hemorrhages are commonly observed while cotton wool spots and macular hard exudates are seldom found. In more advanced cases, retinal arterioles may be narrowed. The contralateral eye (i.e. the non-affected eye) usually reveals a small cup-to-disc ratio or a “disc at risk.”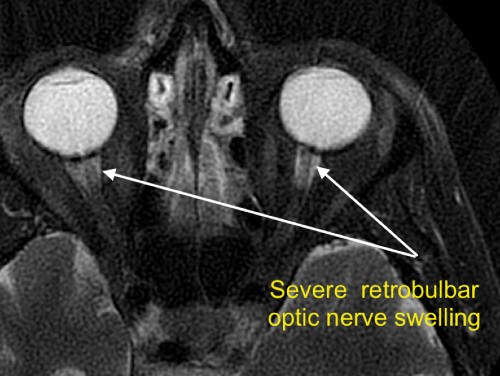
 Although NAION may present with findings similar to that of GCA, a classic presentation of NAION without GCA signs or symptoms may not warrant the usual GCA testing of a complete blood count (CBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). However, if there is any doubt then CBC, ESR, and CRP testing is indicated to rule out GCA. Patients should always be evaluated by a primary-care physician for potential modifiable or treatable risk factors including smoking, sleep apnea, hypertension, diabetes, hyperlipidemia, and other vascular disorders. Neuroimaging is only performed in cases diverging from a typical NAION course such as prolonged optic disc edema, progressing vision loss, or unusual pain. Except in cases of family history or personal clinical evidence, there is no proven value of additional vasculopathic or prothrombotic diagnostic testing (ex. carotid studies, hypercoagulability testing), however homocysteine levels may be tested in patients under the age of 50. Patients with significant hyperhomocysteinemia may be recommended supplementation of vitamins B6, B12, and folic acid and regular monitoring for systemic vasculopathies.
Although NAION may present with findings similar to that of GCA, a classic presentation of NAION without GCA signs or symptoms may not warrant the usual GCA testing of a complete blood count (CBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). However, if there is any doubt then CBC, ESR, and CRP testing is indicated to rule out GCA. Patients should always be evaluated by a primary-care physician for potential modifiable or treatable risk factors including smoking, sleep apnea, hypertension, diabetes, hyperlipidemia, and other vascular disorders. Neuroimaging is only performed in cases diverging from a typical NAION course such as prolonged optic disc edema, progressing vision loss, or unusual pain. Except in cases of family history or personal clinical evidence, there is no proven value of additional vasculopathic or prothrombotic diagnostic testing (ex. carotid studies, hypercoagulability testing), however homocysteine levels may be tested in patients under the age of 50. Patients with significant hyperhomocysteinemia may be recommended supplementation of vitamins B6, B12, and folic acid and regular monitoring for systemic vasculopathies. 

 At the 5 year mark, the probability of NAION development in the fellow eye was 17% in the aspirin group and 20% in the no aspirin group, with no significant difference between the two groups. Overall, studies investigating prophylactic aspirin use to prevent fellow eye involvement fail to demonstrate sufficient evidence to support aspirin therapy, but also demonstrate no increased risk of fellow eye involvement for aspirin users.
At the 5 year mark, the probability of NAION development in the fellow eye was 17% in the aspirin group and 20% in the no aspirin group, with no significant difference between the two groups. Overall, studies investigating prophylactic aspirin use to prevent fellow eye involvement fail to demonstrate sufficient evidence to support aspirin therapy, but also demonstrate no increased risk of fellow eye involvement for aspirin users. %20management%20Ischemic%20Optic%20Neuropathy.jpg?width=597&name=continuous%20positive%20airway%20pressure%20(CPAP)%20management%20Ischemic%20Optic%20Neuropathy.jpg) Additional studies are needed to better understand the role of CPAP usage in preventing NAION for persons with sleep apnea. Other systemic conditions such as hypertension, hypercholesterolemia, and anemia have weaker associations with NAION. Given the vascular and ischemic nature of NAION, it is reasonable to assume that properly treating these conditions could potentially reduce the incidence of NAION as well as other vasculopathic conditions. One study suggested that NAION may be the initial manifestation of hypercholesterolemia in patients younger than 50.
Additional studies are needed to better understand the role of CPAP usage in preventing NAION for persons with sleep apnea. Other systemic conditions such as hypertension, hypercholesterolemia, and anemia have weaker associations with NAION. Given the vascular and ischemic nature of NAION, it is reasonable to assume that properly treating these conditions could potentially reduce the incidence of NAION as well as other vasculopathic conditions. One study suggested that NAION may be the initial manifestation of hypercholesterolemia in patients younger than 50. 
