Damaged optic nerve and retinal cells do have the capacity to provide more function. Vision can be improved significantly through treatment, without encouraging new cell growth or optic nerve fibres to regenerate.
Many diseases and certain medical conditions of the central nervous system can damage the optic nerve causing degeneration of their axons, which subsequently leads to varying forms of eyesight deterioration. The resulting loss of vision depends of severity of damage and spontaneous improvements are unlikely. The axons of retinal ganglion cells (RGCs) which make up the optic nerve do not naturally regenerate, and the ‘gaps’ left along the optic nerve by their degeneration mean that signals from the eye are no longer optimally transferred to the visual cortex of the brain; meaning that our captured images of the world are no longer processed as needed.
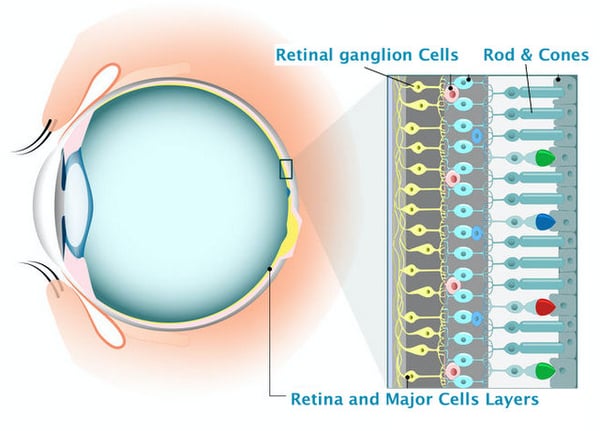 With some medical conditions - like cerebral stroke or peripheral nerves trauma - scientists believe that once the damaged axons of the nerve regenerate, functions improve respectively. With this theory in mind, much work is currently underway in the medical and scientific communities to better understand the mechanisms of optic nerve and RGC regeneration to shed light on the possibility of regenerating damaged cells with a goal to restore lost vision. In parallel, some clinical studies have resulted in proven treatment therapies that can significantly restore vision loss without regeneration; giving hope today to those suffering from optic nerve damage vision loss, while advances in medical science are ongoing. Read on to learn more.
With some medical conditions - like cerebral stroke or peripheral nerves trauma - scientists believe that once the damaged axons of the nerve regenerate, functions improve respectively. With this theory in mind, much work is currently underway in the medical and scientific communities to better understand the mechanisms of optic nerve and RGC regeneration to shed light on the possibility of regenerating damaged cells with a goal to restore lost vision. In parallel, some clinical studies have resulted in proven treatment therapies that can significantly restore vision loss without regeneration; giving hope today to those suffering from optic nerve damage vision loss, while advances in medical science are ongoing. Read on to learn more.
Recent experimental studies related to optic nerve cell regeneration have drawn attention to the fact that vision cannot be effectively restored without first successfully enabling: 1) RGC survival; 2) axon growth to specific targets in the brain; and 3) formation of new functioning synaptic circuits. Preventing RGC damage and subsequent death in the acute phases of many diseases is especially critical for regeneration to occur. Over the last several decades, considerable progress has been made in understanding the molecular pathways involved with RGC survival and death, although these possible therapies have only been studied in animals. Very little has been done to date to apply this knowledge to differing forms of optic neuropathies in humans.
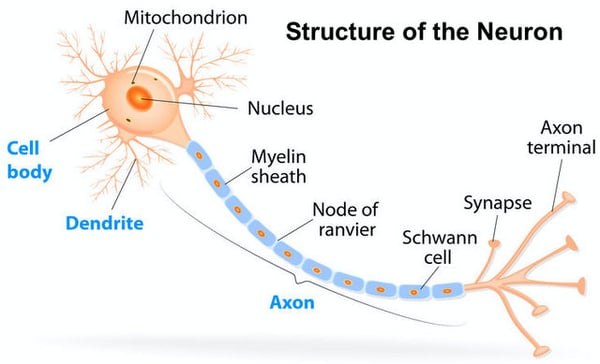 But not only the bodies of RGCs must be regenerated to encourage vision restoration - their axons (or nerve fibres) also require regeneration. In doing so both short and long distance growth must occur, meaning that axons must grow along the damaged area (short regeneration) and they must arrive at their specific target in the brain (long regeneration along the central visual pathways). Encouraging long growth - while preventing abnormal growth and aberrant sprouting - is an even more significant challenge as mechanisms regulating the guidance of regenerating axons are still not well understood. Regenerating axons must also find their specific or propriety targets (i.e., the corresponding neurons in the central visual system that enable visual function), develop synapses and become re-integrated in existing neuronal brain circuits. Although our understanding of the complexities involved in restoring cell function is now more advanced, significant learning is still required to overcome the many challenges inherent in restoring lost vision through RGC and optic nerve regeneration.
But not only the bodies of RGCs must be regenerated to encourage vision restoration - their axons (or nerve fibres) also require regeneration. In doing so both short and long distance growth must occur, meaning that axons must grow along the damaged area (short regeneration) and they must arrive at their specific target in the brain (long regeneration along the central visual pathways). Encouraging long growth - while preventing abnormal growth and aberrant sprouting - is an even more significant challenge as mechanisms regulating the guidance of regenerating axons are still not well understood. Regenerating axons must also find their specific or propriety targets (i.e., the corresponding neurons in the central visual system that enable visual function), develop synapses and become re-integrated in existing neuronal brain circuits. Although our understanding of the complexities involved in restoring cell function is now more advanced, significant learning is still required to overcome the many challenges inherent in restoring lost vision through RGC and optic nerve regeneration.
The complexities of optic nerve regeneration as it relates to vision restoration can be demonstrated through a discussion about glaucoma. Glaucoma – a leading cause of blindness – is a group of eye diseases that progressively and, in many cases, silently damages the optic nerve causing gradual and permanent vision loss. Damage to the optic nerve leads to atrophy (or degeneration) of RGCs and their axons which travel from the retina to the brain; essentially separating the visual cortex in the brain from different parts of the retina. This degeneration of axons - called glaucomatous optic neuropathy - is a result of chronic (and progressive) mechanical, inflammatory and bioenergetic processes. Glaucoma is like other neurodegenerative disorders in that stress at one site (i.e., within the eye) can manifest itself at multiple locations (i.e., in retinal ganglion cells, in both unmyelinated and myelinated segments of the optic nerve, and in neurons in the brain).
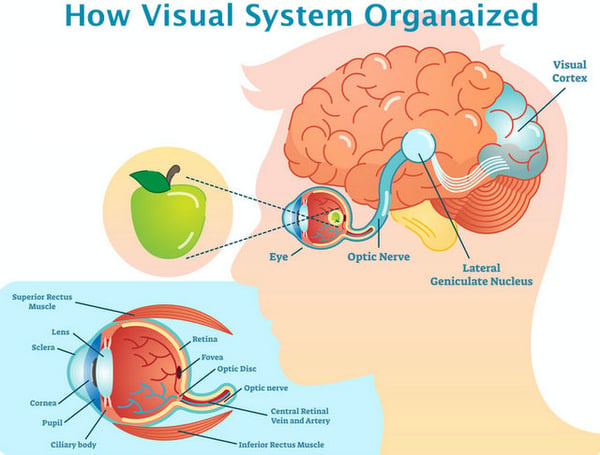
Until recently, it was believed that once damage to the RGC axons begins (i.e., they become thin and atrophic) and the process to convey visual information from the eye to the brain is interrupted there was no going back. (Once up to 40-50% of axons are degenerated, visual function progressively worsens from sight loss to blindness, in very severe cases.) Regeneration of damaged RGC axons was not considered a possibility until 1996 when Berry et al. conducted studies that showed the ability of RGCs in mice to regenerate axons well into the brain. More recently, research in regenerative therapies has resulted in multiple breakthroughs that may unlock the optic nerve’s regenerative potential.
What do Berry’s early studies and the findings of other scientist mean for glaucoma patients? The emerging science – obtained from experimental models – is that if axon function can be repaired early enough, either by reducing intraocular pressure (IOP)-related damage on the axon or by removing the influence of metabolic pathological changes, loss of tissue in glaucoma can be avoided. More recent scientific studies have shown that this same effect might hold true for human glaucoma. (A challenge to confirming this hypothesis is that glaucoma patients would be needed, at appropriate points in their disease’s progression, to study the severity of structural damage prior loss of vision.) The optic nerve and retinal neuronal cells are integral components of the central nervous system and, as mentioned previously, their capacity to regenerate in adult tissues is severely limited. As well, axon degeneration in the optic nerve is not confined to a single area. This means that regenerative strategies potentially need to replace entire lengths of axons or repair axons at multiple points of damage; a significant challenge for developing practical approaches to regenerate fibres of RGCs.
So to progress regenerative science to a point where we can overcome the effects of heightened damage imposed by IOP and partially improve vision in glaucoma patients, we must develop regenerative therapies that consider many challenges. One of them is the necessity to maintain connections between retinal cells and the corresponding neurons in the visual centres of the brain. Not surprisingly, our visual system is very specific in its connections. Although most axons in our brain travel from the eye to special subcortical structures, some target other areas of the brain; all of which combined present us with a full picture of the world around us. As well, cells throughout our visual system map to each other in very specific ways allowing us to receive the highly-organized information we need to see. The richness of our visual experience depends on accurate maintenance of these maps, and we expect regenerative therapies will need to capture the need for restored retinal cells to connect to their corresponding neurons in the brain.
Although significant progress has been made in recent years to better identify and understand regenerative therapies that can help restore vision in patients with optic nerve damage, like those with glaucoma, much more work is needed to translate this emerging science to readily-available treatment therapies. While optic nerve regeneration treatments are not yet a reality for patients, steady advancements are being made of late and hopefully it’s just a matter of time before these remedies are available. That said, patients suffering from vision loss from glaucoma and other conditions causing optic nerve damage don’t have to wait for regenerative strategies to be available. Our Restore Vision Clinic in Berlin has significant experience in both improving vision in glaucoma patients and in slowing down their loss of vision - both of which are achieved without structural regeneration of the damaged optic nerves. Given our scientifically-proven treatments and clinical successes, we are confident in saying that functional restoration of vision is possible today without regeneration. Damaged optic nerve and retinal cells still have a capacity to provide more function. Our experience shows that vision can be improved significantly through treatment, without encouraging new cell growth to regenerate damaged optic nerve fibres.
Since 1993, we have helped improve the vision of over 700 patients suffering from glaucoma. Our patients can expect their vision loss to be slowed - less foggy and clearer sight, increased visual contrast and improvements to field of vision are all benefits of Fedorov Restoration Therapy. These positive outcomes are accomplished through the application of weak electrical current pulses which stimulate partially-damaged retinal cells and improve the conductivity of signals to the brain. Fedorov Therapy can’t replace damaged cells or regenerate optic nerves; instead it increases the functionality of preserved cells on the retina and enhances the activity along optic nerves. Combining electrical stimulation with multimodal brain training enhances the overall activity of the visual system, and leads to functional restoration. Our Fedorov Therapy is a non-invasive and non-surgical way to naturally restore or improve vision, with stable outcomes and without risks of side effects.
Want to learn more? Visit our website to read what other people suffering from glaucoma-related vision loss are saying about this breakthrough treatment.




47 Comments
Click here to read/write comments