Intraocular Pressure (IOP)
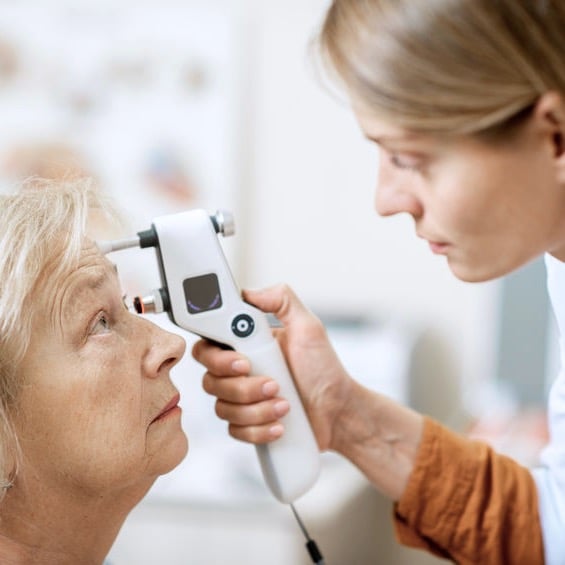
IOP remains the easiest modifiable risk factor, which is partially why glaucoma treatments focus on IOP reduction. Research undeniably demonstrates that high IOP increases risk of glaucoma. Likewise, IOP reduction in NTG also reduces risk of progression.
Age
Even after controlling for all other variables, glaucoma prevalence increases with age.
Ethnicity
Generally, glaucoma is more prevalent in persons of African and Hispanic descent while some secondary glaucoma types are more prevalent in Asian populations. Caucasian populations have a lower glaucoma incidence.
High Myopia
Studies suggest that high myopia is a risk factor for glaucoma, potentially from thinner ocular structures, especially the lamina cribrosa, which become more stretched and less vascularized.
Central Corneal Thickness
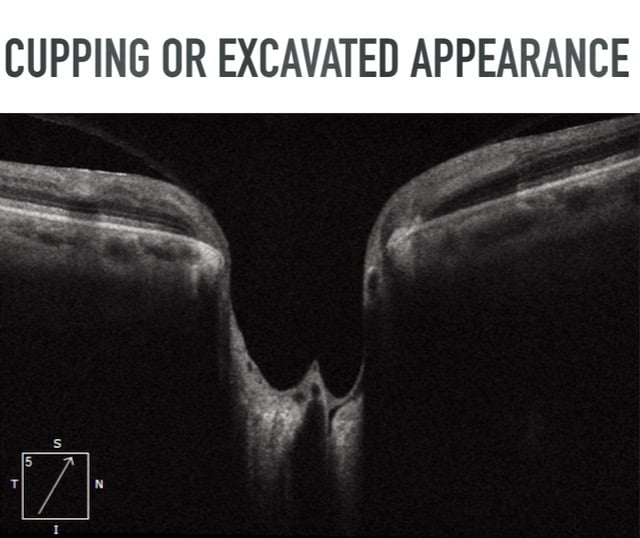
Thinner central corneal thickness is a risk factor, possibly for similar reasons to high myopia, because thin corneas oftentimes are accompanied by a thin lamina cribrosa. Additionally, thin corneas may cause inaccurate IOP measurements, measuring artificially lower than the actual IOP.
Family History
A first-degree relative with glaucoma increases glaucoma risk, especially for siblings. Though several genes are associated with glaucoma, this condition is believed to be polygenic and strongly influenced by the environment.
Systemic Vasculopathic Conditions
Glaucoma occurs frequently in diabetic and hypertensive persons, although some researchers remain skeptical of this association. Both conditions cause vascular dysregulation that may disrupt ONH blood supply. In diabetes, vascular insult arises from damage to the endothelial lining of blood vessels potentially causing ocular vascular dysregulation. Hypertension causes peripheral vascular resistance that may lead to GON. Conversely, excessively low blood pressure (BP) actually decreases OPP of the ONH, which is one concern of anti-hypertensive medications. Aggressive BP reduction in hypertensive patients may predispose a patient to GON. Additionally, sleep apnea induced hypoxia is thought to further glaucoma risk and progression.
Lifestyle
A few initial studies suggest that healthy diet and regular exercise may be preventative for vision loss after a glaucoma diagnosis. Currently such studies are inconclusive. However, if vascular systemic conditions predispose patients to glaucoma, it is plausible that a healthy lifestyle could reduce vascular dysregulation and thus mitigate glaucoma risk.

.jpeg?width=500&name=Glaucoma%20Brain%20degeneration%20Restore%20Vision%20Clinic%20(1).jpeg)
 Note: The information given in this blog are the opinions of the authors and for reader familiarization purposes only. This blog is not intended as a substitute for professional medical advice. Also, the information provided does not replace or abolish any official or legal terms for glaucoma diagnosis, treatment, and management. Authors are not liable for any undesirable consequences or effects related to the information provided in the blog.
Note: The information given in this blog are the opinions of the authors and for reader familiarization purposes only. This blog is not intended as a substitute for professional medical advice. Also, the information provided does not replace or abolish any official or legal terms for glaucoma diagnosis, treatment, and management. Authors are not liable for any undesirable consequences or effects related to the information provided in the blog.
