Retina Overview
.jpeg?width=1280&name=Importe%20-%201%20von%201%20(1).jpeg) The retina is a very thin layer of cells covering the posterior (back) pole of the eyeball that captures external light, converts this light into neural signals, and sends these signals via the optic pathways to the brain. Fascinatingly, the retina is only approximately 0.5mm thick – three sheets of computer paper – yet is the tissue responsible for showing us the world by sending electrical signals to the brain throughout our lives. When functioning properly the human retina is capable of resolving approximately 576 megapixels. Compare that with the latest iPhone with its touted camera of 12 megapixels. Artificial intelligence and camera lenses remain light-years away from reaching the impeccable clarity of the human retina.
The retina is a very thin layer of cells covering the posterior (back) pole of the eyeball that captures external light, converts this light into neural signals, and sends these signals via the optic pathways to the brain. Fascinatingly, the retina is only approximately 0.5mm thick – three sheets of computer paper – yet is the tissue responsible for showing us the world by sending electrical signals to the brain throughout our lives. When functioning properly the human retina is capable of resolving approximately 576 megapixels. Compare that with the latest iPhone with its touted camera of 12 megapixels. Artificial intelligence and camera lenses remain light-years away from reaching the impeccable clarity of the human retina.
Despite its thinness, the retina is composed of 10 distinct layers. Light travels through the thickness of the retina before stimulating photopigment inside the photoreceptors (specialized cells called cones and rods). These photoreceptors in the posterior (back) retina photo-chemically convert photons of light into neural signals through a process called phototransduction. These signals travel from the posterior (outer) to the anterior (inner) retina and are modified and integrated along the way by various cells in other retinal layers. The photochemical transduction process is tremendously complicated and still not fully understood. Phototransduction requires enormous amounts of oxygen and nutrients.
 In fact, the retina is known to be the most metabolically active tissue in the body and photoreceptors require more oxygen than any other human cell. The combination of photoreceptors demanding a massive oxygen supply with the relatively low vascular nature of the outer retina substantially increases the risk of cell damage by hypoxic injury. This delicate, transparent tissue of exceedingly complicated anatomy also requires substantial metabolic support, which predisposes the retina to dysfunction with even the slightest abnormalities.
In fact, the retina is known to be the most metabolically active tissue in the body and photoreceptors require more oxygen than any other human cell. The combination of photoreceptors demanding a massive oxygen supply with the relatively low vascular nature of the outer retina substantially increases the risk of cell damage by hypoxic injury. This delicate, transparent tissue of exceedingly complicated anatomy also requires substantial metabolic support, which predisposes the retina to dysfunction with even the slightest abnormalities.
The process of creating, using, disposing, and regenerating metabolites used in phototransduction is part of the visual cycle. Small retinal or metabolic anomalies may disrupt the visual cycle and the phototransduction process thereby hindering these electrical signals from reaching the brain via the optic nerve. Inhibition of any part of this process may result in visual impairment. This may be a general reduction of vision, vision loss in specific areas of the retina (and therefore field of vision), or diminished types of functional vision such as color or contrast vision.
Retinal Structure
As mentioned above, despite the astonishing thinness of the retina, it is composed of 10 distinct layers. Adequate phototransduction and sufficient conveyance of visual signals is accomplished through highly specialized retinal anatomy. The retina is shaped like a circular disc between 30-40mm in diameter, with the center 6mm being considered the central retina (macula). Only the macula, which accounts for 3-5% of the total retina, is capable of achieving 20/20 vision. The remaining retina has far less image resolution capability.
A protective yellow-pigmented layer consisting of xanthophyll carotenoids (lutein and zeaxanthin) called the macula lutea covers the macula and filters out short-wavelength (blue) light. The centermost part of the macula, termed the fovea, is responsible for sharp, central vision. An anatomical pit is located at the center of the fovea giving the retina its thinnest area at only approximately 200-220 microns. This foveal contour occurs due to retinal layers being displaced laterally. Several retinal layers disappear in the fovea making room for densely packed cone-photoreceptors, which enhance visual sharpness (image). Interesting, the fovea is avascular (it has no direct blood vessels to supply cells). With the exception of the foveal pit, the macula is generally thicker than the peripheral retina due to increased density of cone photoreceptors.
The most peripheral portion of the retina is called the ora serrata, radially located approximately 21mm from the fovea. Photoreceptor-wise, cones dominate the central retina while rods are found in the peripheral retina. The neural-retina oftentimes refers to the first 9 layers of the retina, while the retinal pigment epithelium (RPE) is considered a supportive layer for the neural-retina. The RPE is not involved in photochemical conversion of light into electrical signals, but rather, protects and supports the neural-retina, especially through the blood-retinal barrier. The retina is transparent in nature to allow for light to pass through the anterior (front) retinal layers before reaching the photoreceptors in the posterior (back) retina. The architecture of the human retina demonstrates remarkably clear anatomical and functional distinction.
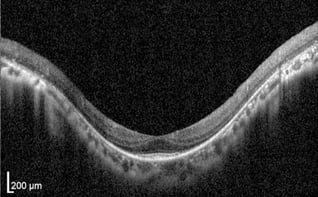
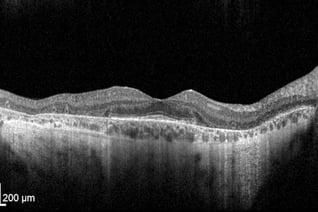
Retinal OCT scan. Undamaged retina.
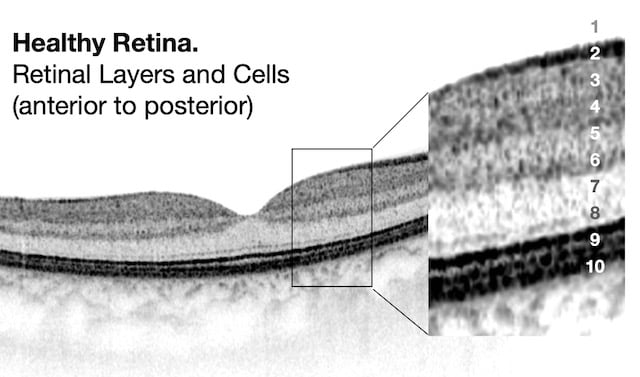
OCT Abbreviation:
1. Internal limiting membrane (ILM) – footplate of Muller cells
2. Retinal nerve fiber layer (RNFL) – axons of ganglion cells
3. Ganglion cell layer (GCL) – nuclei of ganglion cells
4. Inner plexiform layer (IPL) – synapse between bipolar cells with ganglion and amacrine cells
5. Inner nuclear layer (INL) – nuclei of amacrine, bipolar, and horizontal cells
6. Outer plexiform layer (OPL) – synapses between rods/cones with bipolar and horizontal cells
7. Outer nuclear layer (ONL) – cells bodies (nuclei) of rods and cones
8. External limiting membrane (ELM) – Muller cell endplates
9. Photoreceptor layer (PL) – contains outer segments of rods and cones
10. Retinal pigment epithelium (RPE) – nourishes and supports neural-retina, absorbs stray light with pigment
Retinal Cells and Function
The human visual system is a remarkably intricate arrangement beginning with allowing external light stimuli to project through the retina and be subsequently absorbed by photopigments within the photoreceptors. These photons are transduced into a biochemical message before becoming an electrical signal that is modulated by ensuing retinal cells as this neural signal travels to the anterior (front) of the retina and then to the optic nerve via retinal ganglion cell axons. The optic nerve is the part of the visual pathway that connects retinal cells with the brain. In the primary visual cortex of the brain (left and right occipital lobes) (image), this neural retinal signal is processed and analyzed, completing the transformation of previous photon light signals into detailed and practical visual information.
Research indicates this visual signal is processed and refined as it travels through the retina to the visual cortex. Some of this modulation occurs via a vertical excitatory pathway of the retina, while other alterations transpire through two lateral inhibitory pathways. Horizontal cells in the outer retina supply inhibitory feedback of the photoreceptors while amacrine cells of the inner retina deliver inhibitory feedback of the bipolar and ganglion cells. Here is a brief summary of the functions of specific retinal cell types.
Layers of retinal cells
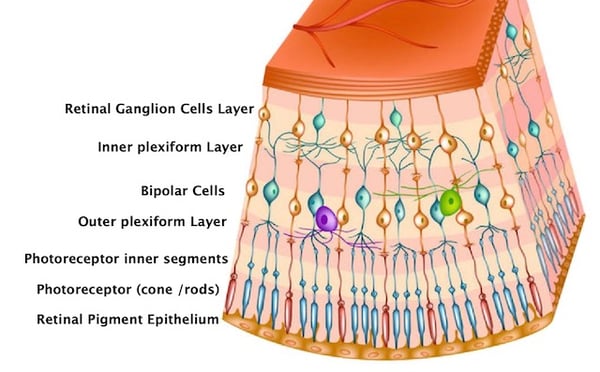
Rods and cones: capture and phototransduce light into bioelectrical signals
Bipolar: receive signals from photoreceptors and transmit signals to the inner retina
Ganglion*: transmit neural signals to the brain via the optic nerve
Horizontal: modulate and integrate neural signals, lateral inhibition to OPL
Amacrine: modulate and integrate neural signals, lateral inhibition to IPL
Interplexiform: modulate and integrate neural signals, lateral inhibition to OPL and IPL
Muller: structure and functional support
*a subtype of retinal ganglion cells called intrinsically photosensitive retinal ganglion cells (ipRGCs) are a third type of photoreceptor which are stimulated by blue light and modulate circadian rhythm, pupil responses, migraines, and certain moods.
Retinal Blood Supply
As previously mentioned, the retina is one of the most metabolically active tissues in the human body and requires tremendous oxygen consumption to properly function. This metabolically demanding tissue requires an extensive, dual vascular supplying network of vessels which both originate from the ophthalmic artery.
-2.jpeg?width=500&name=Importe%20-%201%20von%201%20(2)-2.jpeg)
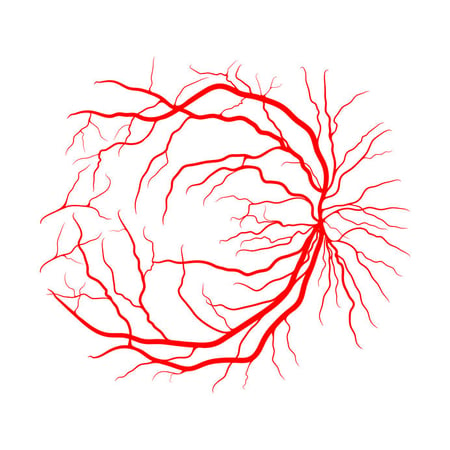
Eye fundus image. Both source of retinal blood supply are seen.
Note: Patient has severe atrophy of choroid - it's why choroidal vessels well seen.
Only the inner portion of the retina is actually supplied by retinal blood vessels. This anterior two-thirds of the retina receives its blood supply from the central retinal branch of the ophthalmic artery. This central retinal artery enters along the optic nerve and branches into four distinct arterial arcades at anterior surface of the retina, which are observable via ophthalmoscopy. These arteries branch into arterioles traveling through the RNFL whose shadows are perceptible using optical coherence tomography (OCT) imaging. These arterioles form an interconnecting plexus of three sets of capillaries between the inner RNFL, outer RNFL and GCL, and the INL. Despite only accounting for two-thirds of the thin retinal tissue, the inner retina receives 20-30% of total ocular blood flow. Although this blood supply is primarily for the inner retina, oxygen may extend as far as the inner photoreceptors during times of intense oxygen demand. A similar and parallel system of venules and veins exists for drainage.
-3.jpeg?width=450&name=Importe%20-%201%20von%201%20(1)-3.jpeg) The outer portion of the retina is supplied through the choroid and not through retinal circulation (image). This posterior third of the retina is supplied through a “large-capillary lake” layer called the choriocapillaris, which is located directly behind the retina and is fed by the choroid. By unit weight, the choroid has the greatest blood flow of any tissue in the human body, the highest perfusion rate of any vascular bed, and receives 65-85% of total ocular blood flow. The choroid primarily receives blood supply from the long and short ciliary branches of the ophthalmic artery, but the anterior ciliary arteries contribute to a lesser degree. In addition to supplying the outer third of the retina, the choroid also supplies the RPE and part of the optic nerve head. Since the outer retina is avascular, the choroid provides oxygen and nutrients to the photoreceptors through free diffusion. The one or two vortex veins in each retinal quadrant drain the choroidal blood.
The outer portion of the retina is supplied through the choroid and not through retinal circulation (image). This posterior third of the retina is supplied through a “large-capillary lake” layer called the choriocapillaris, which is located directly behind the retina and is fed by the choroid. By unit weight, the choroid has the greatest blood flow of any tissue in the human body, the highest perfusion rate of any vascular bed, and receives 65-85% of total ocular blood flow. The choroid primarily receives blood supply from the long and short ciliary branches of the ophthalmic artery, but the anterior ciliary arteries contribute to a lesser degree. In addition to supplying the outer third of the retina, the choroid also supplies the RPE and part of the optic nerve head. Since the outer retina is avascular, the choroid provides oxygen and nutrients to the photoreceptors through free diffusion. The one or two vortex veins in each retinal quadrant drain the choroidal blood.
Retinal blood supply evaluation
 The health of the retinal blood supply can be evaluated using several different ophthalmological instruments. Fundus photography provides similar views of the retinal blood supply as dilated fundus examination, but in two dimensions rather than three. These images display the retinal architecture supplying blood flow to the inner retina, but not clear views of the underlying choroid (image). Optical coherence tomography (OCT) provides cross-sectional scans of the retinal layers and choroid. Although not particularly helpful in visualizing specific retinal vessel architecture, the aftermath of unhealthy retinal vessels is quite apparent, such as with fluid edema or new blood vessel growth (neovascularization). OCT angiography (OCTA) on the other hand is a new, powerful tool for analyzing retinal and choroidal intravascular blood flow in real time that may reveal vessel leakage, capillary dropout, or ischemia. Angiography (FA) is the traditional method of assessing retinal and choroidal circulation during different stages of flow. Although being extremely effective in diagnosing circulation abnormalities, FA is accompanied with certain risks such as nausea, vomiting, headaches, fatigue, and even anaphylaxis. OCTA is not a complete substitute for FA, but OCTA does provide a quick, painless, and non-invasive alternative to traditional FA in certain cases.
The health of the retinal blood supply can be evaluated using several different ophthalmological instruments. Fundus photography provides similar views of the retinal blood supply as dilated fundus examination, but in two dimensions rather than three. These images display the retinal architecture supplying blood flow to the inner retina, but not clear views of the underlying choroid (image). Optical coherence tomography (OCT) provides cross-sectional scans of the retinal layers and choroid. Although not particularly helpful in visualizing specific retinal vessel architecture, the aftermath of unhealthy retinal vessels is quite apparent, such as with fluid edema or new blood vessel growth (neovascularization). OCT angiography (OCTA) on the other hand is a new, powerful tool for analyzing retinal and choroidal intravascular blood flow in real time that may reveal vessel leakage, capillary dropout, or ischemia. Angiography (FA) is the traditional method of assessing retinal and choroidal circulation during different stages of flow. Although being extremely effective in diagnosing circulation abnormalities, FA is accompanied with certain risks such as nausea, vomiting, headaches, fatigue, and even anaphylaxis. OCTA is not a complete substitute for FA, but OCTA does provide a quick, painless, and non-invasive alternative to traditional FA in certain cases.
The Retina: An Extension of the Brain
The brain and retina exhibit anatomical, functional, and immunological similarities that may explain the link between retinal and neurological degeneration.
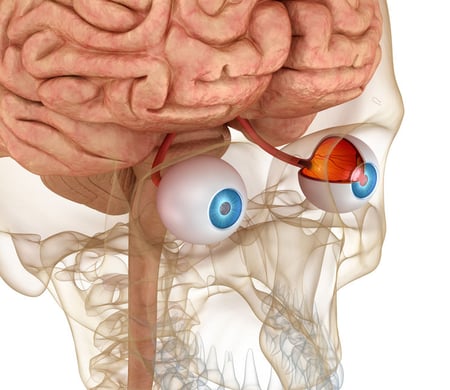
- Several common neurodegenerative diseases (ex. Parkinson’s and Alzheimers) have retinal manifestations indicating the eye may be a ‘window’ into brain health
- The same neurodegenerative mechanisms found in brain disorders are also observed in retinal pathologies
- Ease of accessibility and structure makes retinal research a useful tool to better understand the brain
- Research indicates the potential role of ocular imaging as a non-invasive method to help diagnosis brain disorders
- Further research may focus on the possibility of therapeutic approaches being simultaneously beneficial for ocular and neurological disorders
Evidence is now emerging suggesting associations between retinal atrophy and brain atrophy of the primary visual cortex. These findings demonstrate structural and functional alterations within the retina and brain for certain retinal and neurological conditions. Furthermore, retinal input may elicit neurodevelopmental reorganization in the primary visual cortex. For instance, Leber Hereditary Optic Neuropathy – known to cause retinal ganglion cell death – has been associated with reduced gray matter in the primary visual cortex of the brain. Glaucoma and age-related macular degeneration may also have corresponding neurological degeneration. Conversely, retinal changes have been observed in patients with Parkinson’s, Alzheimer’s, multiple sclerosis, and even strokes.
Summary
In every facet, the retinal proves to be notoriously complicated – intricate retinal architecture, elaborate cellular function, convoluted blood supply, and even associations with neurological degeneration and function. Sharp visual function is dependent on immaculate retinal structure and copious oxygen and nutrient availability. For this reason, visual function may be disturbed with even the slightest retinal irregularity. Retinal pathology is defined by which abnormality occurs, the subsequent retinal alteration, and the ensuing visual changes.

.jpeg?width=1280&name=Importe%20-%201%20von%201%20(1).jpeg) The retina is a very thin layer of cells covering the posterior (back) pole of the eyeball that captures external light, converts this light into neural signals, and sends these signals via the optic pathways to the brain. Fascinatingly, the retina is only approximately 0.5mm thick – three sheets of computer paper – yet is the tissue responsible for showing us the world by sending electrical signals to the brain throughout our lives. When functioning properly the human retina is capable of resolving approximately 576 megapixels. Compare that with the latest iPhone with its touted camera of 12 megapixels. Artificial intelligence and camera lenses remain light-years away from reaching the impeccable clarity of the human retina.
The retina is a very thin layer of cells covering the posterior (back) pole of the eyeball that captures external light, converts this light into neural signals, and sends these signals via the optic pathways to the brain. Fascinatingly, the retina is only approximately 0.5mm thick – three sheets of computer paper – yet is the tissue responsible for showing us the world by sending electrical signals to the brain throughout our lives. When functioning properly the human retina is capable of resolving approximately 576 megapixels. Compare that with the latest iPhone with its touted camera of 12 megapixels. Artificial intelligence and camera lenses remain light-years away from reaching the impeccable clarity of the human retina. In fact, the retina is known to be the most metabolically active tissue in the body and photoreceptors require more oxygen than any other human cell. The combination of photoreceptors demanding a massive oxygen supply with the relatively low vascular nature of the outer retina substantially increases the risk of cell damage by hypoxic injury. This delicate, transparent tissue of exceedingly complicated anatomy also requires substantial metabolic support, which predisposes the retina to dysfunction with even the slightest abnormalities.
In fact, the retina is known to be the most metabolically active tissue in the body and photoreceptors require more oxygen than any other human cell. The combination of photoreceptors demanding a massive oxygen supply with the relatively low vascular nature of the outer retina substantially increases the risk of cell damage by hypoxic injury. This delicate, transparent tissue of exceedingly complicated anatomy also requires substantial metabolic support, which predisposes the retina to dysfunction with even the slightest abnormalities.

-2.jpeg?width=500&name=Importe%20-%201%20von%201%20(2)-2.jpeg)
-3.jpeg?width=450&name=Importe%20-%201%20von%201%20(1)-3.jpeg) The outer portion of the retina is supplied through the choroid and not through retinal circulation (image). This posterior third of the retina is supplied through a “large-capillary lake” layer called the choriocapillaris, which is located directly behind the retina and is fed by the choroid. By unit weight, the choroid has the greatest blood flow of any tissue in the human body, the highest perfusion rate of any vascular bed, and receives 65-85% of total ocular blood flow. The choroid primarily receives blood supply from the long and short ciliary branches of the ophthalmic artery, but the anterior ciliary arteries contribute to a lesser degree. In addition to supplying the outer third of the retina, the choroid also supplies the RPE and part of the optic nerve head. Since the outer retina is avascular, the choroid provides oxygen and nutrients to the photoreceptors through free diffusion. The one or two vortex veins in each retinal quadrant drain the choroidal blood.
The outer portion of the retina is supplied through the choroid and not through retinal circulation (image). This posterior third of the retina is supplied through a “large-capillary lake” layer called the choriocapillaris, which is located directly behind the retina and is fed by the choroid. By unit weight, the choroid has the greatest blood flow of any tissue in the human body, the highest perfusion rate of any vascular bed, and receives 65-85% of total ocular blood flow. The choroid primarily receives blood supply from the long and short ciliary branches of the ophthalmic artery, but the anterior ciliary arteries contribute to a lesser degree. In addition to supplying the outer third of the retina, the choroid also supplies the RPE and part of the optic nerve head. Since the outer retina is avascular, the choroid provides oxygen and nutrients to the photoreceptors through free diffusion. The one or two vortex veins in each retinal quadrant drain the choroidal blood.  The health of the retinal blood supply can be evaluated using several different ophthalmological instruments. Fundus photography provides similar views of the retinal blood supply as dilated fundus examination, but in two dimensions rather than three. These images display the retinal architecture supplying blood flow to the inner retina, but not clear views of the underlying choroid (image). Optical coherence tomography (OCT) provides cross-sectional scans of the retinal layers and choroid. Although not particularly helpful in visualizing specific retinal vessel architecture, the aftermath of unhealthy retinal vessels is quite apparent, such as with fluid edema or new blood vessel growth (neovascularization). OCT angiography (OCTA) on the other hand is a new, powerful tool for analyzing retinal and choroidal intravascular blood flow in real time that may reveal vessel leakage, capillary dropout, or ischemia. Angiography (FA) is the traditional method of assessing retinal and choroidal circulation during different stages of flow. Although being extremely effective in diagnosing circulation abnormalities, FA is accompanied with certain risks such as nausea, vomiting, headaches, fatigue, and even anaphylaxis. OCTA is not a complete substitute for FA, but OCTA does provide a quick, painless, and non-invasive alternative to traditional FA in certain cases.
The health of the retinal blood supply can be evaluated using several different ophthalmological instruments. Fundus photography provides similar views of the retinal blood supply as dilated fundus examination, but in two dimensions rather than three. These images display the retinal architecture supplying blood flow to the inner retina, but not clear views of the underlying choroid (image). Optical coherence tomography (OCT) provides cross-sectional scans of the retinal layers and choroid. Although not particularly helpful in visualizing specific retinal vessel architecture, the aftermath of unhealthy retinal vessels is quite apparent, such as with fluid edema or new blood vessel growth (neovascularization). OCT angiography (OCTA) on the other hand is a new, powerful tool for analyzing retinal and choroidal intravascular blood flow in real time that may reveal vessel leakage, capillary dropout, or ischemia. Angiography (FA) is the traditional method of assessing retinal and choroidal circulation during different stages of flow. Although being extremely effective in diagnosing circulation abnormalities, FA is accompanied with certain risks such as nausea, vomiting, headaches, fatigue, and even anaphylaxis. OCTA is not a complete substitute for FA, but OCTA does provide a quick, painless, and non-invasive alternative to traditional FA in certain cases. 
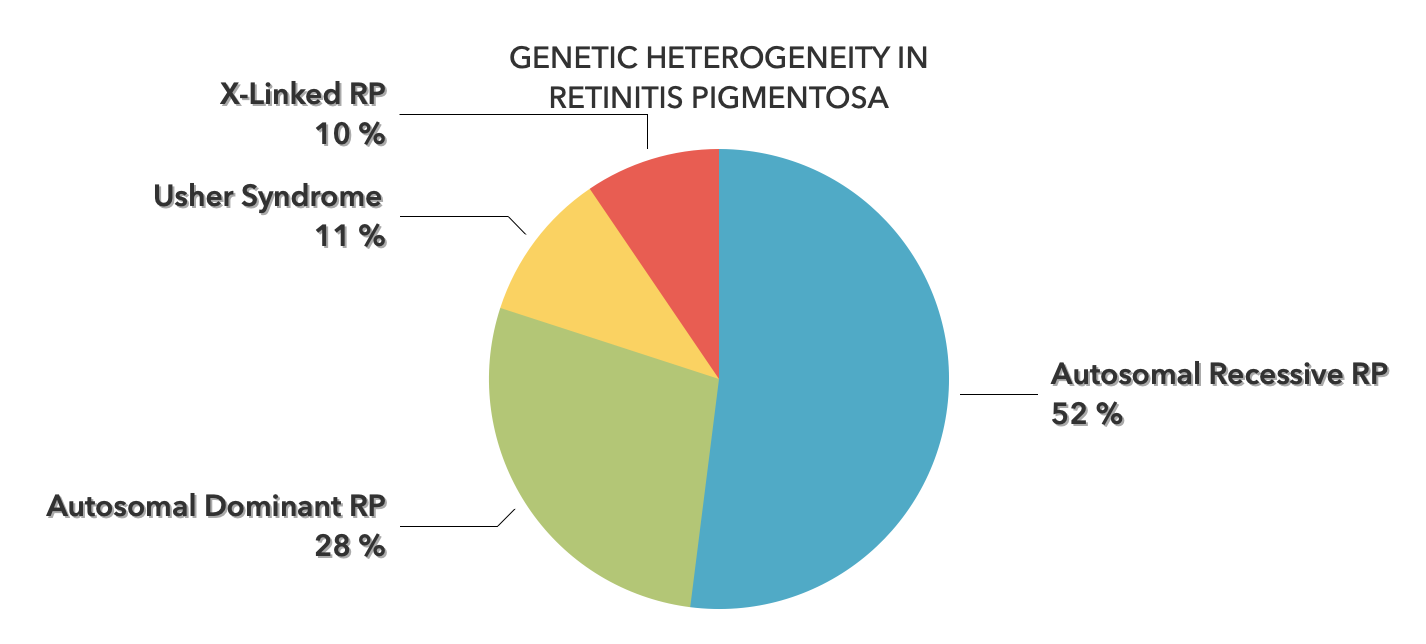
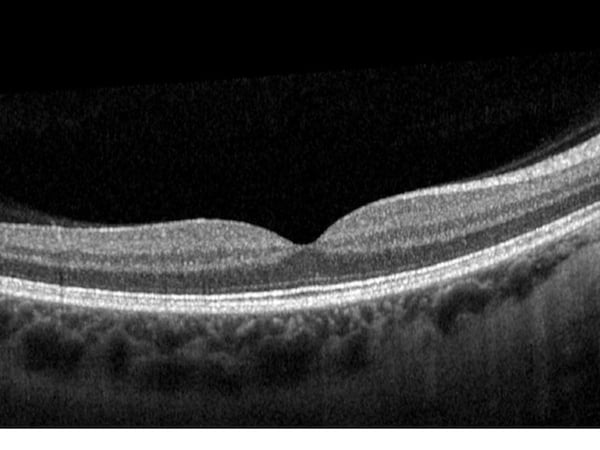
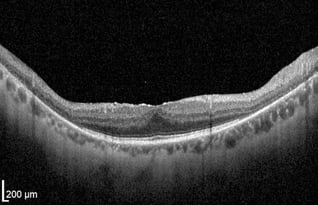
-2.jpeg?width=562&name=Importe%20-%201%20von%201%20(3)-2.jpeg)
%20OCT%20exam%20Restore%20Vision%20Clinic%20.jpg?width=600&name=Congenital%20Stationary%20Night%20Blindness%20(CSNB)%20OCT%20exam%20Restore%20Vision%20Clinic%20.jpg)
-2.jpeg?width=586&name=Importe%20-%201%20von%201%20(4)-2.jpeg)
-2.jpeg?width=580&name=Importe%20-%201%20von%201%20(5)-2.jpeg)

-2.jpeg?width=900&name=Importe%20-%201%20von%201%20(6)-2.jpeg)
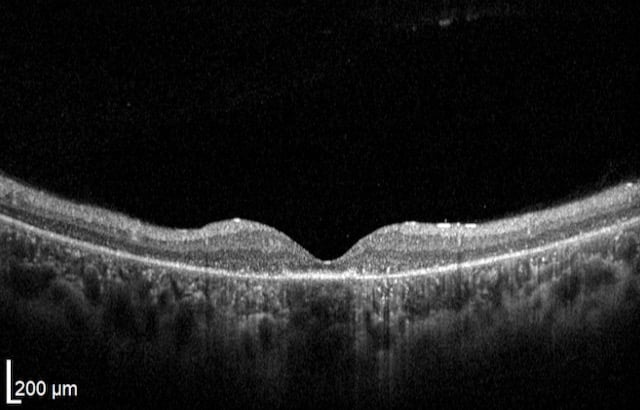
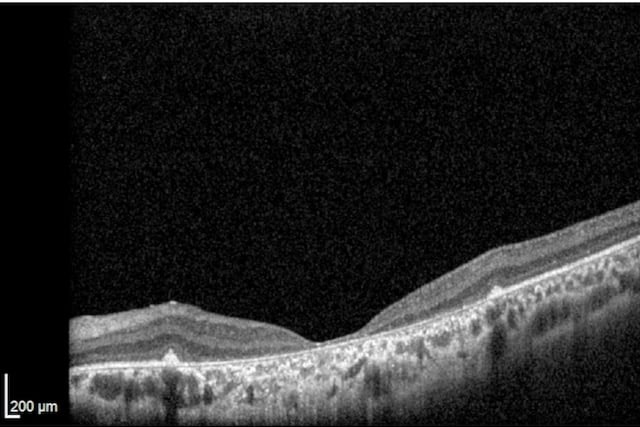
 Unlike peripheral vision, central vision loss does not always occur in retinitis pigmentosa. Central vision, if affected at all, remains relatively preserved until later in the disease course, despite the possibility of some abnormal structural or pigmentary changes. Not all genetic forms of RP cause diminished central vision and the severity of central vision deterioration is highly variable. Worsening central vision may begin in middle age due to cone photoreceptor dysfunction in the macula. When central vision is affected, very rarely is all vision completely lost. More than half of RP patients 45 years of age or older are capable of seeing at least 20/40 in one eye. From the same study, only one fourth of RP patients see 20/200 or worse with both eyes, and only 0.5% are completely blind in both eyes.
Unlike peripheral vision, central vision loss does not always occur in retinitis pigmentosa. Central vision, if affected at all, remains relatively preserved until later in the disease course, despite the possibility of some abnormal structural or pigmentary changes. Not all genetic forms of RP cause diminished central vision and the severity of central vision deterioration is highly variable. Worsening central vision may begin in middle age due to cone photoreceptor dysfunction in the macula. When central vision is affected, very rarely is all vision completely lost. More than half of RP patients 45 years of age or older are capable of seeing at least 20/40 in one eye. From the same study, only one fourth of RP patients see 20/200 or worse with both eyes, and only 0.5% are completely blind in both eyes.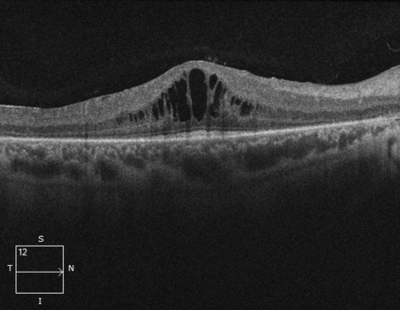 In macular-involving RP, one of the most common complications is cystoid macular edema (CME). Estimated to appear in up to 50% of RP patients, CME occurs when fluid cysts form in the center of the retina and cause retinal swelling or edema. Although painless, CME causes distorted and blurry central vision. Other macular complications with RP include epiretinal membranes (ERM) and macular holes. ERM formation occurs in approximately 36% of RP cases. Macular holes distort and reduce central vision. ERMs may range from benign to causing moderate visual distortion, but may lead to other macular problems. Although non-RP-induced CME is normally only observed in older patients (especially post-operatively), RP-induced CME is found in all age groups. Some studies suggest, but do not confirm, that CME is more prevalent in autosomal dominant RP. Other studies suggest a higher prevalence of CME amongst female RP patients. However the high occurrence of CME in the entire RP population suggests no specific subtype predilection.
In macular-involving RP, one of the most common complications is cystoid macular edema (CME). Estimated to appear in up to 50% of RP patients, CME occurs when fluid cysts form in the center of the retina and cause retinal swelling or edema. Although painless, CME causes distorted and blurry central vision. Other macular complications with RP include epiretinal membranes (ERM) and macular holes. ERM formation occurs in approximately 36% of RP cases. Macular holes distort and reduce central vision. ERMs may range from benign to causing moderate visual distortion, but may lead to other macular problems. Although non-RP-induced CME is normally only observed in older patients (especially post-operatively), RP-induced CME is found in all age groups. Some studies suggest, but do not confirm, that CME is more prevalent in autosomal dominant RP. Other studies suggest a higher prevalence of CME amongst female RP patients. However the high occurrence of CME in the entire RP population suggests no specific subtype predilection. Since peripheral eyesight is the predominant form of vision affected with RP,
Since peripheral eyesight is the predominant form of vision affected with RP, %20(1).png?width=350&name=Alle%20Fotos%20-%201%20von%201%20(2)%20(1).png) As with all retinal and optic nerve conditions,
As with all retinal and optic nerve conditions,  Modern electrophysiology aids in the diagnosis and management of RP by providing quantitative and objective measurements of retinal function. The most useful
Modern electrophysiology aids in the diagnosis and management of RP by providing quantitative and objective measurements of retinal function. The most useful  The most common treatment intervention historically has been Vitamin A supplementation. Vitamin A is essential for photoreceptor function and is utilized poorly in RP. Some studies suggest Vitamin A supplementation results in slower decline in vision loss and slower ERG a-wave reduction (representing photoreceptor function). Although the results are mixed, several studies indicate daily supplementation with 15,000 IUs of Vitamin A may slow vision deterioration in adults with classic RP.
The most common treatment intervention historically has been Vitamin A supplementation. Vitamin A is essential for photoreceptor function and is utilized poorly in RP. Some studies suggest Vitamin A supplementation results in slower decline in vision loss and slower ERG a-wave reduction (representing photoreceptor function). Although the results are mixed, several studies indicate daily supplementation with 15,000 IUs of Vitamin A may slow vision deterioration in adults with classic RP. Before beginning any supplementation, including Vitamin A, several items must be considered:
Before beginning any supplementation, including Vitamin A, several items must be considered: Note: The information given in this blog are the opinions of the authors and for reader familiarization purposes only. This blog is not intended as a substitute for professional medical advice. Also, the information provided does not replace or abolish any official or legal terms for glaucoma diagnosis, treatment, and management. Authors are not liable for any undesirable consequences or effects related to the information provided in the blog.
Note: The information given in this blog are the opinions of the authors and for reader familiarization purposes only. This blog is not intended as a substitute for professional medical advice. Also, the information provided does not replace or abolish any official or legal terms for glaucoma diagnosis, treatment, and management. Authors are not liable for any undesirable consequences or effects related to the information provided in the blog.