Introduction
Sleep is a biological necessity critical for physical, mental, and emotional health. Poor sleep quality and duration has been linked with a myriad of medical conditions and disorders affecting many organ systems of our body. Insufficient sleep is classified by the Center for Disease Control as a global epidemic. Disordered sleep affects millions of people and is responsible for decreased productivity caused by daytime sleepiness, days lost from work, and accidents at work and home. There are more than 60 known sleep disorders, which are grouped into six major categories: Insomnia, Sleep Related Breathing Disorders, Central Disorders of Hypersomnolence, Circadian Rhythm Sleep-Wake Disorders, Parasomnias, and Sleep Related Movement Disorders.
More common of then - obstructive sleep apnea (OSA) plays is more significant role in health related complications and diseases. The range of severity for OSA is widespread and highly variable. While certain cases are infrequent and a minor nuisance affecting sleep, other cases are severe, instigating ocular and systemic complications. Most notably, OSA has been linked with cardiovascular and neurological conditions including heart disease, arrhythmias, hypertension, diabetes, stroke, and dementia.
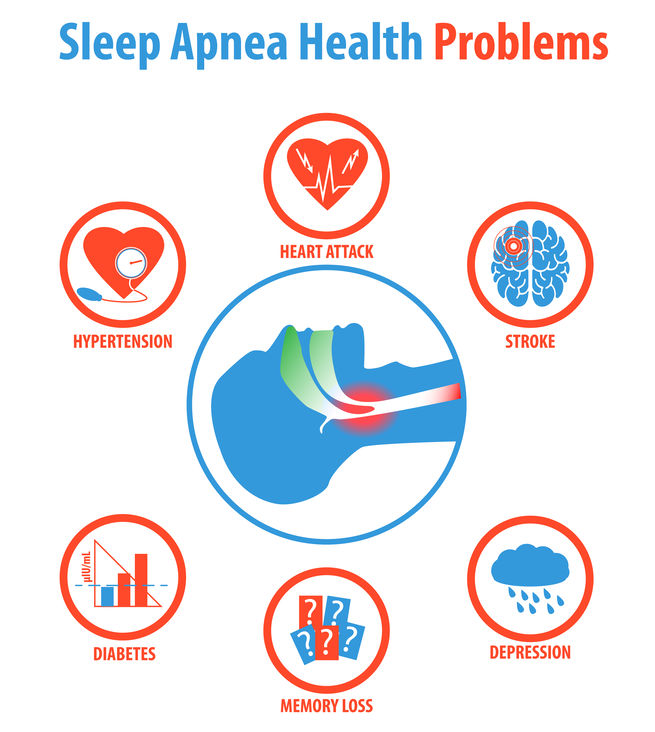
OSA is characterized by episodes of repeated upper airway obstruction during sleep resulting in nighttime hypoxia, frequent arousal, and sleep fragmentation. Breathing interruptions from OSA may occur up 100 times during the night resulting in appreciably diminished oxygen intake.
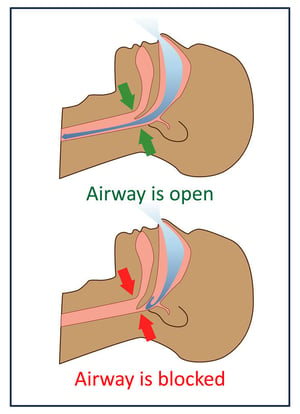 Sleep apnea pathophysiology is characterized by interruption of respiration during sleep. Apnea refers to complete cessation of breathing while partial cessation from blockage is called hypopnea. Both apnea and hypopnea events typically last between 30 and 60 seconds but up to two minutes or longer in severe cases leading to prominent oxygen desaturation. Blood oxygen levels can drop below 85% which is dangerously low when occurring for 30-45 seconds or longer. Increased CO2 (carbon dioxide) concentrations activate part of the central nervous system triggering awakeness (arousal). These frequent arousals and fragmented sleep leads to many daytime symptoms.
Sleep apnea pathophysiology is characterized by interruption of respiration during sleep. Apnea refers to complete cessation of breathing while partial cessation from blockage is called hypopnea. Both apnea and hypopnea events typically last between 30 and 60 seconds but up to two minutes or longer in severe cases leading to prominent oxygen desaturation. Blood oxygen levels can drop below 85% which is dangerously low when occurring for 30-45 seconds or longer. Increased CO2 (carbon dioxide) concentrations activate part of the central nervous system triggering awakeness (arousal). These frequent arousals and fragmented sleep leads to many daytime symptoms.
Symptoms of OSA can be divided into two groups: a) related to airway obstruction with broken breathing and b) the results of temporal ischemia (low oxygenation) in different organs and the brain. Patients in the first group experience excessive snoring, choking, gasping for air, increased nighttime bathroom trips, dry or sore throat, and interrupted sleep. Poor chronic brain oxygenation causes symptoms of morning headaches, poor daytime concentration, and daytime drowsiness. Signs of irritability, restlessness, forgetfulness, behavioral instability, anxiety, and even depression may be observed in severe cases.
OSA prevalence increases linearly with age and is estimated to occur in approximately 9% of women and 24% of men. Furthermore, a survey from the National Sleep Foundation revealed that 31% of men and 21% of women are at high risk for OSA. Risk factors for OSA include high body mass index, aging, smoking, alcohol consumption, sedentary lifestyle, sleeping in a supine position, large neck circumference, nasal obstruction, and increased pharyngeal tissue.
Sleep Apnea and Nonarteritic Anterior Ischemic Optic Neuropathy
The visually devastating condition of NAION is strongly associated with OSA. It is well known that brain and retina are of particular concern as they consume the highest concentration of oxygen in the body and are therefore especially susceptible to hypoxic conditions. OSA may provoke optic nerve damage through multiple conditions, the most notable being nonarteritic anterior ischemic optic neuropathy (NAION), which will be discussed in detail below.
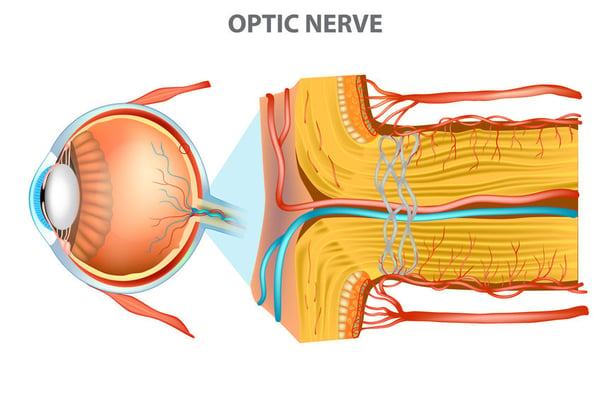
Aptly described in the name, NAION is an optic neuropathy occurring from ischemia to the anterior portion of the optic nerve. The optic nerve blood supply is convoluted and layer dependent. The anterior optic nerve affected in NAION shares the same blood supply as the inner retina — it derives from its own retinal blood circulation and it essentially auto-regulated apart from systemic blood circulation, which has the capability of autoregulation. Conversely, the posterior optic nerve is oxygenated through choroidal vasculature and regulated systemically. As mentioned before, optic nerve is highly sensitive and easily damaged in cases of oxygen deprivation. Therefore, blood supply is important to remember when considering the pathogenesis of NAION.
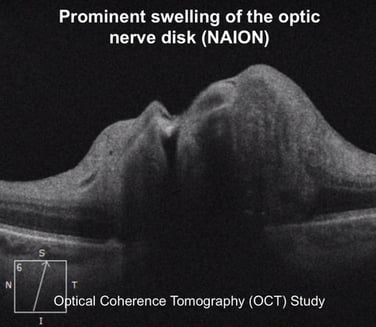
Nonarteritic Anterior Ischemic Optic Neuropathy
Clinically NAION is characterized as an acute, painless, moderate to severe vision loss usually in one eye. Approximately 73% of those afflicted notice vision loss upon awakening in the morning which suggests a pathological sleep related mechanism, possibly nocturnal arterial hypotension. Vision may be lost centrally, peripherally, or both and to varying degrees of severity. For this reason, central visual acuity may range from 20/20 to no light perception. Dyschromatopsia, or color blindness, may also be present and typically corresponds to the degree of visual acuity loss. Two requirements for a diagnosis of NAION include presence of a relative afferent pupillary defect (APD) and optic disc edema at the onset of visual symptoms. Optic disc edema may be localized or diffuse and associated with hyperemia while nerve fiber layer hemorrhages may be visualized along the optic disc margins. Although generally non-progressive, vision loss from NAION is typically considered irreversible and severe. A subset of patients (30-45%) notice a decline in vision in the 30 days following the ischemic event while approximately 43% of patients have mild/moderate spontaneous improvements within 6 months.
While some NAION cases are idiopathic in nature, known risk factors exist including small cup/disc ratio of the optic nerve (commonly referred to as a “disc at risk”), diabetes, arterial hypertension, nocturnal hypotension, atherosclerosis, arteriosclerosis, hypercholesterolemia, and OSA. Approximately 70-80% of patients with NAION have been diagnosed with OSA and those with NAION are five times more likely to have OSA. NAION is the most common form of acute optic neuropathy in persons above the age of 50, but far less likely to occur before the age of 50. Though relatively uncommon, the annual incidence for those above the age of 50 is as high as 82/100,000 persons/year while the incidence for all ages is only about 3.72/100,000 persons/year. Men and women are affected equally and the majority of cases occur in those of Caucasian descent.
Two of the forefront researchers investigating the relationship between OSA and NAION, Bixler and Mojon, concluded after a series of studies that the prevalence of OSA amongst NAION patients is “real and significant”. Likewise, Palombi and colleagues conducted subsequent research and determined “NAION was nearly always associated with sleep apnea”. Young et al demonstrated the relative risk of OSA occurrence among NAION patients is 4.9 times greater compared to the overall population. Surveys conducted further supported this connection, revealing that people with NAION are 2.62 times more likely to have survey scores consistent with OSA. Stein and colleagues discovered that OSA patients not treated with positive airway pressure have a 16% increased risk of a NAION attack.
Though the relationship between OSA and NAION is well established in literature, the exact mechanism triggering this event is still unknown. Nocturnal blood pressure variations are commonly implicated as the culprit in the development of NAION, of which small cup to disk ratio optic nerves (“discs at risk”) are particularly vulnerable. While inconclusive, several studies suggest that patients taking antihypertensive medications may be at increased risk, as such medications significantly lower nighttime systolic blood pressure, causing a larger percent difference between daytime and nighttime blood pressure when compared to normal diurnal blood pressure variations. Various theories attempt to elucidate the precise mechanism of OSA provoking an NAION occurrence. These theories may be divided into three categories: mechanical, vascular, and direct.
Mechanical Theory: The mechanical theory hypothesizes that the hypoxic episodes from OSA result in elevated blood pressure and ICP thus triggering optic nerve head (ONH) edema and ischemia via mechanical stress and focal compression of the optic nerve and/or blood vessels supplying the optic nerve. Raised intracranial pressure may occur from increased central venous pressure and increased cerebrovascular volume, raised systemic arterial blood pressure, or hypercapnia induced cerebral vasodilation.
Vascular Theory: Fluctuations in blood pressure are commonly observed in OSA from intermittent hypoxia triggering erratic sympathetic activation and inconsistent hemodynamic conditions. From this irregularity, apnea-induced vascular dysregulation of the blood vessels supplying the ONH is conceivable. The hypoxia-reoxygenation pattern from OSA may metabolically stress the autoregulatory vascular system to the optic nerve, thus inducing vascular insufficiency. Furthermore, the vascular endothelium of the optic nerve supplying vessels is likely damaged from oxidative stress due to a fluctuating metabolic state.
Direct Theory: An alternative and plausible supposition is simply direct optic nerve damage secondary to prolonged hypoxia or frequent hypoxic conditions known to occur in cases of OSA. In an attempt to understand which occurred initially, circulatory impairment or optic disc edema, Arnold conducted histopathological and fluorescein angiographic studies. His results indicate circulatory impairment precedes optic disc edema, thus suggesting a vascular mechanism, but does not dismiss mechanical compression of blood vessels triggering this vascular insult.
Second eye at risk.
Roughly 15-24% of patients experience a subsequent ischemic episode in the contralateral eye thus making proper management of risk factors vital as this mitigates the risk of vision loss in the other eye. Though not fully supported by research thus far, the detrimental visual consequences of a contralateral NAION attack indicate aggressive treatment of underlying systemic conditions especially in cases of diabetes, arterial hypertension, nocturnal hypotension, atherosclerosis, arteriosclerosis, hypercholesterolemia, and most notably OSA. Proper treatment of OSA is critical to prevent damage to the healthy eye as well as further damage to the affected eye. Even though the incidence of a second distinct ischemic attack of the same eye is only approximately 3.6% in the three-year period following the initial ischemic event, measures should be taken to prevent further vision loss of the affected eye as well.
Not treating OSA increases the risk of an NAION attack. Given the severity of vision loss associated with an NAION event, every precaution should be taken to prevent a similar incident in the fellow eye as this may result in devastating visual impairment and diminished quality of life.
Lastly, several studies demonstrate that patients with NAION may be at greater risk of developing ischemic strokes and myocardial infarctions. Therefore, referral to a cardiologist or general physician is indicated. Prompt referral and proper management of NAION is critical, as this condition may not only lead to vision loss, but life-threatening systemic complications as well. Modifiable risk factors and applicable lifestyle changes should also be addressed.
Other Known Ophthalmic Associations of OSA
Known ophthalmic complications associated with OSA include Floppy Eyelid Syndrome (FES), keratoconus (KC), glaucoma, papilledema, central serous chorioretinopathy (CSCR), retinal vein occlusions (RVO), and – most notably – NAION. OSA induced hypoxia is also recognized to worsen existing retinal conditions such as diabetic retinopathy.
Predominantly seen in overweight males, FES is characterized by flaccid and loose upper eyelids. FES manifests as eyelid droopiness, chronic eye irritation and discomfort, dryness, and conjunctival redness. The prevalence of FES in OSA patients ranges from 2-32%, while obesity in OSA patients is estimated to be 60-70%, and the prevalence of obesity in FES populations ranges from 43-92%. Therefore, it is currently unclear as to which associations are directly or indirectly occurring. However, a causal relationship is uncertain, as several studies have also revealed a relationship between OSA and FES independent of body weight.
Preliminary evidence suggests a possible correlation between OSA and a corneal degeneration called keratoconus (KC). KC is characterized as a progressive steepening and thinning of the cornea resulting in ectasia or bulging of the cornea. Recent studies found the prevalence of OSA to be approximately 18-19% among patients with KC. However, this may be an indirect association as obesity is both a risk factor for KC and OSA.
 While any association between OSA and glaucoma remains unproven, researchers theorize that vascular and mechanical factors in OSA may lead to glaucomatous optic neuropathy. In addition to a high prevalence of glaucoma amongst OSA patients, diagnostic criteria associated with glaucoma such as thinning of the retinal nerve fiber layer and visual field loss has been tied to OSA. Additionally, treatment of OSA has been shown to effectively reduce the risk of glaucoma. Hypoxia from repeated and prolonged airway obstruction causes optic nerve damage, oxidative stress, increased inflammation, vascular resistance, increased intracranial pressure (ICP), and autonomic dysfunction. Increased ICP and obesity-inducing elevated intraocular pressure (IOP) may damage the lamina cribrosa and trabeculum of the optic nerve through mechanical force. These vascular and mechanical consequences of OSA may lead to glaucomatous optic neuropathy and subsequent loss of peripheral and central vision.
While any association between OSA and glaucoma remains unproven, researchers theorize that vascular and mechanical factors in OSA may lead to glaucomatous optic neuropathy. In addition to a high prevalence of glaucoma amongst OSA patients, diagnostic criteria associated with glaucoma such as thinning of the retinal nerve fiber layer and visual field loss has been tied to OSA. Additionally, treatment of OSA has been shown to effectively reduce the risk of glaucoma. Hypoxia from repeated and prolonged airway obstruction causes optic nerve damage, oxidative stress, increased inflammation, vascular resistance, increased intracranial pressure (ICP), and autonomic dysfunction. Increased ICP and obesity-inducing elevated intraocular pressure (IOP) may damage the lamina cribrosa and trabeculum of the optic nerve through mechanical force. These vascular and mechanical consequences of OSA may lead to glaucomatous optic neuropathy and subsequent loss of peripheral and central vision.
 Hypercapnia induced cerebral venous dilation and raised venous pressure from apneic episodes of OSA is believed to raise ICP, which may manifest in the eye as papilledema or swelling of the optic nerve. This sustained increase in ICP may obstruct retrograde axonal transport causing this optic nerve swelling, which if prolonged may lead to vision loss. Several case reports have demonstrated the cessation of papilledema related visual symptoms upon treatment of OSA.
Hypercapnia induced cerebral venous dilation and raised venous pressure from apneic episodes of OSA is believed to raise ICP, which may manifest in the eye as papilledema or swelling of the optic nerve. This sustained increase in ICP may obstruct retrograde axonal transport causing this optic nerve swelling, which if prolonged may lead to vision loss. Several case reports have demonstrated the cessation of papilledema related visual symptoms upon treatment of OSA.
CSCR, a serous detachment of the neurosensory retina, is generally seen in male patients with elevated stress levels and Type A personalities. Although far from conclusive, studies have postulated a relationship between OSA and CSCR suggesting an underlying mechanism of increased cortisol levels and sympathetic activity resulting in increased choroidal permeability and vascular endothelial dysfunction. Treatment of OSA has been shown to accelerate the recovery of CSCR.
Multiple studies and case reports suggest an association between OSA and retinal vein occlusions (RVO) explained by the potential mechanism of hypoxia induced blood circulation mitigation and elevation nocturnal IOP. Prognosis of an RVO, or “eye stroke” in laymen’s terms, is highly variable but can result in significant monocular vision loss.
Management of OSA
Early diagnosis and intervention in OSA is crucial as many ocular and system complications are irreversible, but preventable. OSA is highly treatable and improvement of symptoms is common. Additionally, inherent ocular and systemic risks from OSA may be lessened as blood vessels have demonstrated remodeling in as little as 3-4 months following treatment.
Achieving optimal body weight is the priority when managing OSA as this significantly reduces episodes, symptoms, and complications. Avoidance of smoking, alcohol consumption, and sedatives is also highly recommended. First line medical intervention for OSA includes use of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP) devices.
%20management%20Ischemic%20Optic%20Neuropathy.jpg?width=845&name=continuous%20positive%20airway%20pressure%20(CPAP)%20management%20Ischemic%20Optic%20Neuropathy.jpg)
This positive pressure to the upper airway prevents collapse of the pharynx during sleep, enabling steady oxygen intake through uninterrupted breathing. Wake promoting agents may be used to treat residual excessive daytime sleepiness in conjunction with CPAP use. Though less common, surgical management to stiffen airway passages or remove the tonsils, uvula, or soft palate are considered secondary treatment options in cases involving intolerance or failure of positive airway pressure devices. Recent strides have been made to create smaller and lighter positive airway devices.
Studies have demonstrated the use of CPAP during sleep alleviates apneic episodes, lessens sleep fragmentation, mitigates hemodynamic fluctuations, improves blood pressure, and decreases the risk of cardiac arrhythmias. It is reasonable to assume that treatment of OSA may significantly reduce the risk of ocular related complications, especially NAION.
General physicians, sleep specialists, and pulmonologists should be cognizant of the ocular risks accompanying OSA. Regular eye examinations are prudent and necessary. Though not confirmed by research at this point, proper management of OSA likely significantly mitigates the risk of ocular complications, especially NAION. 
Likewise, optometrists and ophthalmologists should identify high-risk OSA patients and refer them to sleep specialists for polysomnography, as severe systemic risks associated with OSA are well established. This could include patients with FES, KC, glaucoma, papilledema, CSCR, RVO, and NAION – especially if the patient is overweight, reports daytime fatigue, snores loudly, or gasps/chokes during sleep.
Conclusion
Occasionally the relationship between certain medical conditions is not intuitive, which may be the case with obstructive sleep apnea and nonarteritic anterior ischemic optic neuropathy. OSA has extensive implications both systemically and ocularly. Educating practitioners and patients of these associations improves patient care and outcomes. Specifically, understanding the strong connection between OSA and NAION helps practitioners achieve prompt and appropriate referrals. Through proper management of OSA and underlying systemic conditions we can help prevent loss of vision and other systemic complications.
FEDOROV RESTORATION THERAPY
Most patients suffering from NAION have been told that their vision loss is irreversible and there is no treatment for an injured optic nerve. Patients are often told that these nerves have no capacity to regenerate, and therefore the patient’s sight can never be recovered or improved. This leaves patients feeling hopeless and without options to restore their vision — but this is far from true. Years of research and practical work has shown that vision loss caused by NAION can be partially reversed resulting in significant improvement in quality of life and visual function. Our clinic in Berlin offers Fedorov Restoration Therapy which is a painless, straightforward therapy designed to improve the function of retinal cells and the entire optic nerve. Treatment involves application of weak electrical current pulses targeting partially-damaged retinal cells to improve the conductivity of signals to the brain thus enhancing visual signals through the damaged optic nerve. The Fedorov Restoration Therapy interdisciplinary approach bridges ophthalmology and neurology, eye and brain, combining the two in order to achieve spectacular results for visually impaired patients afflicted by optic nerve disorders. This is an innovative and completely non-invasive, non-surgical method that improves and restores vision naturally with stable results and with no risk of side effects. If you or a loved one are afflicted by visual impairment, especially from optic nerve damage, Fedorov Restoration Therapy might be the perfect treatment for you.
Blog prepared in cooperation with Kaleb Abbott, O.D., M.S.
References
- Chattu VK, Manzar MD, Kumary S, Burman D, Spence DW, Pandi-perumal SR. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare (Basel). 2018;7(1)
- Lee YC, Wang JH, Huang TL, Tsai RK. Increased Risk of Stroke in Patients With Nonarteritic Anterior Ischemic Optic Neuropathy: A Nationwide Retrospective Cohort Study. Am J Ophthalmol. 2016;170:183-189.
- Li J, Mcgwin G, Vaphiades MS, Owsley C. Non-arteritic anterior ischaemic optic neuropathy and presumed sleep apnoea syndrome screened by the Sleep Apnea scale of the Sleep Disorders Questionnaire (SA-SDQ). Br J Ophthalmol. 2007;91(11):1524-7.
- Archer EL, Pepin S. Obstructive sleep apnea and nonarteritic anterior ischemic optic neuropathy: evidence for an association. J Clin Sleep Med. 2013;9(6):613-8.
- Iyer SR, Iyer RR, Parikh V, Ramchandani S. Obstructive Sleep Apnea and Ophthalmic Disorders-Clinical Implications. J Assoc Physicians India. 2018;66(4):55-9.
- Stuart, Annie. “Obstructive Sleep Apnea and the Eye: The Ophthalmologist’s Role.” American Academy of Ophthalmology, 5 May 2016, https://www.aao.org/eyenet/article/obstructive-sleep-apnea-eye-ophthalmologist-s-role.
- Santos M, Hofmann RJ. Ocular Manifestations of Obstructive Sleep Apnea. J Clin Sleep Med. 2017;13(11):1345-1348.
- Liguori C, Palmieri MG, Pierantozzi M, et al. Optic Nerve Dysfunction in Obstructive Sleep Apnea: An Electrophysiological Study. Sleep. 2016;39(1):19-23.





2 Comments
Click here to read/write comments